Corrosion
Corrosion is the
term used to describe the process of the surface of metal objects getting
covered by oxides (or) other salts of the metal. It is basically defined as a
natural process that causes the transformation of pure metals into undesirable
substances when they react with substances like water or air. This reaction
causes damage and disintegration of the metal starting from the portion of the
metal exposed to the environment and spreading to the entire bulk of the metal.
Corrosion is
usually an undesirable phenomenon since it negatively affects the desirable
properties of the metal. For example, iron is known to have good tensile
strength and rigidity (especially alloyed with a few other elements). However,
when subjected to rusting, iron objects become brittle, flaky, and structurally
unsound.
Factors Affecting Corrosion
·
Exposure of the metals to air
containing gases like CO2, SO2, SO3 etc.
·
Exposure of metals to moisture
especially salt water
·
Presence of impurities like salt
(eg. NaCl).
·
Temperature: An increase in
temperature increases corrosion.
·
Presence of acid in the atmosphere:
acids can easily accelerate the process of corrosion.
Types of corrosion
Corrosion occurs in many ways depending upon the attack of the
metal, by the surrounding medium. There are two types of corrosion
·
Dry corrosion
·
Wet Corrosion
Dry corrosion
Dry corrosion occurs when metals come in direct contact with atmospheric
gases like O2, CO2 etc. Dry corrosion is hence often
referred to as direct chemical corrosion or atmospheric corrosion. The rate of
corrosion is faster especially near industrial areas as atmospheric air
contains more corrosive gases in industrial area. Dry corrosion is then further
subdivided in
·
Oxidation corrosion
·
Corrosion due to other gases
Oxidation corrosion
Oxidation corrosion is a type of dry corrosion where corrosion
takes place mainly because of presence of O2. Oxygen present in air
directly reacts with metal either at low or high temperatures in the absence of
moisture.
Mechanism of oxidation corrosion
Oxidation corrosion is brought about by direct action of oxygen on
metals by forming oxide film. The mechanism of oxide film formation can be
represented by the following reactions:
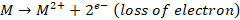
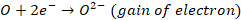
Net reaction,
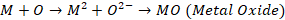
In above reactions, the electrons are transferred from metal atom
to oxygen. Metal losses electrons while oxygen accepts electrons forming their
respective ions. These two types of ions combine together to form metal oxide
layer. Nature of the oxide film formed on the surface of metal plays an
important part in oxidation corrosion process. For further oxidation to
continue a) the metal ion must diffuse outwards or b) oxide ion must diffuse
inwards. Former occurs more readily as because of the smaller size of the metal
ion.
In oxidation corrosion, the nature of the oxide film plays an
important role. It may be
a) Stable A stable layer of
metal oxide is formed which is impervious and this protects the metal from
further corrosion. Eg :- Al, Sn. "If the volume of the oxide film is
greater than the volume of the meta surface, then the oxide layer is protective
and non-porous. On the other hand," if the volume of the metal oxide is
less than the volume of the metal, then the layer is non-protective and
porous". This is known as Pilling-Bed worth rule.
b) Unstable The oxide layer
decomposes back to metal and oxygen.
c) Volatile Oxide layer vaporises
as soon as it is formed. Then by the metal surface is exposed for further
attack. For example, molybdenum oxide
d) Porous Oxide layer has
porous or cracks, so oxygen has access to the underlying metal. Hence, corrosion
continues.
Wet corrosion or electro-chemical corrosion
This type of corrosion occurs at a solid-liquid interface when the
metals are in contact with moist air or any liquid medium. If two dissimilar
metals are dipped in a solution, the solution act as a conducting medium
between them. One of the two metals acts as the anode and the other as a
cathode.
At anode: Oxidation
(Corrosion) takes place i.e. 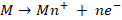
At cathode: Reduction takes
place, Where electrons are consumed.
Corrosion of an anode is based on how electrons are consumed at
cathode.
At cathode consumption of electron takes place either by
liberation or evolution of hydrogen or by absorption of oxygen. It is also
known as immersed corrosion as it occurs in metal when they are immersed or
dipped in the same solution.
Galvanic corrosion or Bimetallic corrosion- When two dissimilar metals are connected in an electrolyte, the
metal higher in electrochemical series undergoes corrosion. Eg:- Zn and Cu. Zn
acts as anode and is protected & Cu acts as cathode.
Concentration Cell corrosion- It is due to electrochemical attack on the metal surface, exposed
to an electrolyte of varying concentrations or of varying aeration. This may be
the result of local differences in metal ion concentrations.
Differential aeration corrosion- The most common type of concentration cell corrosion occurs when
different parts of the metal are exposed to different O2 or air concentration.
Less oxygenated part acts as anode and undergoes corrosion, whereas more
oxygenated parts acts as cathode.
Protection of metal from corrosion
The various protective measures include
·
Modification of the environment
·
Cathodic protection
·
Use of protective metal coating
Modification of environment
In this method, the metals are protected from corrosion either by
removal of corrosion stimulants or by the use of inhibitors. A corrosion
inhibitor is a substance which when added in small quantities to the aqueous
corrosive environment and effectively decreases the corrosion of the metal.
Cathodic protection
The metal to be protected is forced to behave like cathode, there
by corrosion does not occur.
There are two types of cathodic protection
Sacrificial anodic protection: In this method, the metallic structure (to be protected) is
connected to more anodic material. So, that the corrosion is concentrated at
more active metal. The more active metal sacrifices itself by corrosion and
protects the structure (cathode). The sacrificial anode are Zn, Mg, Al. This
method is mainly used for protection of underground pipeline, cables, and
marine structures.
Impressed current cathodic protection: In this method, an impressed current is applied in opposite
direction to nullify the corrosion current and convert the corroding metal from
anode to cathode. The impressed current is derived from a direct current source
like a battery. Sufficient D.C current is applied to an insoluble anode, buried
in the soil and connected to the metallic structure to be protected. This type
of cathodic protection is applied to open water-box coolers, water-tanks,
buried oil or water pipes etc.
Application of protective coatings
This method is most commonly used method to prevent metal from
corrosion. As its name suggests, a coating is applied over metal so it doesn’t
get corroded. It can be classified as
Coating of less active metal: Less active metals also known
as noble metals protect the base metal from corrosion due to their inactivity.
The protection by less active metal is satisfactory as long as the coating is
perfect. A break or crack in coating may facilitate the formation of electrolytic
cells due to which, more active base metal undergoes corrosion rapidly.
Coating of more active metal: Coating of more active metals like zinc, aluminium, cadmium
protect the base metal from corrosion. In this case, base metal act as a
cathode and coating metal becomes an anode. Thus, the two metals being in
contact with the surrounding medium form a galvanic cell in which cathode
remains un-attacked, while the anode corrodes.
Methods of applying metal coating
The protective metallic coating can be made by the following
different methods
·
Hot dipping: Galvanizing and Tinning
·
Metal spraying
·
Electroplating
·
Metal cladding
·
Diffusion coating: Colorizing,
Chromizing and Sherardizing
Hot dipping
Hot dipping method is based on the process of dipping the base
metal in a molten coating metal covered by a molten flux layer. The flux cleans
the surface of base metal and prevents the oxidation of molten coating metal.
The base metal is of higher melting point like iron, steel, etc and the coating
metal is lower melting point then base metal. Hot dipping is generally done by
two methods
Galvanising
Galvanizing, or galvanization, is a manufacturing process where a
coating of zinc is applied to steel or iron to offer protection and prevent
rusting. In this method, steel or iron is dipped in a molten pool of zinc that
maintains a temperature of around 860°F (460 °C). This molten bath begins a
metallurgical bond between the zinc and the receiving metal. After the metal is
pulled from the bath, it reacts to being exposed to the atmosphere, and the
pure zinc mixes with oxygen to form zinc oxide. The zinc-oxide further reacts
to carbon dioxide and forms zinc carbonate, which makes up the final protective
coating on the material.
Steps involved in hot dip galvanizing
·
The iron or steel article to be
galvanized is first cleaned with dilute sulphuric acid to remove oxide layer
and impurities.
·
It is then dipped in a bath of zinc
ammonium chloride solution and then allowed to dry.
·
The sheet is then dipped molten
zinc.
·
It is then passed under a roller to
make the coating uniform and remove excess of molten zinc.
·
It is then heated at a temperature
of 600o C to 700o C and then cooled down slowly.
Advantages
·
Low installation and maintenance
cost
·
Long endurance
Disadvantages
·
Galvanized coating makes containers
unusable for edible stuffs.
Tinning
Tinning is the process of coating iron with molten tin, which can
be used for edible applications. Tinning is the process of making tinplate,
which consists of sheets of iron or steel that have been thinly coated with tin
by being dipped in a molten bath of that metal. Hence the process is more
precisely described as hot-dipped tin plating. This is done in order to prevent
the iron from rusting.
Steps involved in process of tinning
·
The iron or steel article to be
galvanized is first cleaned with dilute sulphuric acid to remove oxide layer
and impurities.
·
It is then dipped in a bath of zinc
ammonium chloride solution and then allowed to dry.
·
The sheet is then dipped molten tin.
·
It is then dipped in a suitable
vegetable oil, to protect the hot tin coated surface against oxidation.
·
It is then passed under a roller to
make the coating uniform and remove excess of molten tin.
Difference between galvanization and tinning
|
Galvanizing
|
Tinning
|
|
Process of coating steel with a thin coat of TIN to prevent it
from corrosion
|
Process of covering iron or steel with a thin coat of ZINC to
prevent it from rusting.
|
|
Tin protects the base metal iron, from corrosion due to its
noble nature and higher corrosion resistance.
|
Zinc protects iron sacrificially. Since it is more
electro-positive than iron and does not permit iron to pass into the
solution.
|
|
Tin protects underlying iron till the coat is intact. Any break
in coating causes rapid corrosion of iron.
|
In galvanized articles zinc continues to protect the underlying
iron by galvanic cell action even if the coating of zinc is broken at any
place
|
|
Tin coated containers and utensils can be used for storing any
food stuff as tin is non-toxic and protects metal from corrosion.
|
Galvanized containers cannot be used for storing acidic
foodstuffs as zinc reacts with food acid forming poisonous compounds.
|
|
Ideal temperature is around 250 degree C.
|
Ideal temperature is around 450 degree C.
|
|
Zinc chloride is used as flux.
|
Ammonium chloride is used as flux.
|
Metal spraying
Metal spraying, or metallizing, is the process of coating a
surface with metal or alloy using spray equipment. It is used to guard metals
from corrosion, employing zinc or aluminium as basic spray materials. The
typical hard-facing materials used in metal spraying include cobalt, nickel
with a little amount of chromium, and manganese chrome.
Steps involved in metal spraying
·
The spraying gun consists of a duct
for compressed air. The coating metal which needs to be sprayed is fed into the
gun in form of wire.
·
The metal wire gets melted using
compressed air to form a fine spray.
·
The spray can be directed to the
surface where fine molten droplets rapidly solidify and form the coating. This
coating protects the base metal from corrosion.
Advantages
Spraying can be applied to non-metal as well.
Uneven surfaces can be easily protected using metal spraying.
Disadvantages
The process is not so effective as the coating may be porous and
less adherent.
Electroplating
Electroplating is basically the process of plating a metal onto
the other by hydrolysis mostly to prevent corrosion of metal or for decorative
purposes.
Steps involved in electroplating
·
The surface which needs to be coated
is first cleaned and suspended into the electrolyte and made as cathode. The
anode consists of the pure metal whose coating is desired on the article.
·
The electrolyte generally consists
of a salt solution of the coating metal.
·
When electric current is passed
through the solution, the metal ions from electrolyte get deposited on the
article. The equivalent amount of anode gets dissolved in the form of ions and
passed into the electrolyte.
·
A thin layer of superior coating
metal is obtained on the cathode.
Advantages
·
Improves wear resistance.
·
Improves the thickness of the metal
surface.
·
Enhancing the electrical
conductivity like plating a copper layer on an electrical component.
·
Minimizing Friction.
Sherardizing
Sherardizing is a zinc diffusion coating process, which uses zinc
vapor to form zinc alloys with the base material. For sherardizing it is
necessary to have the source (Zn powder) close to the coating surface, because
of the low partial pressure of zinc.
Steps involved in Sherardizing
·
The iron articles to be coated are
first cleaned.
·
The articles are then packed with
zinc dust and zinc powder oxide in a steel drum.
·
The steel drum is provided with
electrical heating arrangement to raise the temperature. The steel drum is
rotated by means of a motor.
·
During this period, zinc gets
diffused into iron forming Fe-Zn alloy at the surface which protects the iron
surface from corrosion.
Advantages
·
The coating of metal is uniform
·
No change in dimension of articles.
Metal Cladding
Metal cladding is a type of protective coating, where the
protective material such as metal powder or foil is bonded to a substrate by
applying heat and/or pressure.
Steps involved in metal cladding
·
The base metal to be protected is
sandwiched between two sheets of coating metal.
·
This cladded metal is then passed
through two heavy rollers maintained at high temperature.
·
The sandwiched metal becomes
cathodic with respect to base metal so that the electrolytic protection is
provided. All corrosion resistance metal can be used as a cladding material.
Advantages
·
It increases the strength of the
base metal.
·
The cladding metal also provide
electrolytic protection to base metal.
Disadvantages
·
Only plain surfaces can be protected
using metal cladding.
·
Base metal cannot be completely
prevented from corrosion using metal cladding.
Paints
Paints are mechanical dispersion mixture of one or more pigments
in a non-volatile film forming material (usually a thinner). Paints are used
for decorative purpose as well as protective coating over metal. Paint needs
time to dry out. To accelerate the drying, small amount of dryers are also
added to the paint.
Characteristics of a good paint
Fluidity: Paint should be
fluid enough so that it easily spreads over the entire surface to be protected.
Coverage: Paints should have
good covering capability.
Durability: A good paint should be durable and long lasting
Adherence: A good paint should adhere to the surface to which it has been
applied.
Appearance: The paint film should look glossy and shinny.
Flexibility: The paint
should not get cracked on drying
Constituents of paints
Paints is a composition of various materials. But some
constituents are essential for a paint. They are discussed below:
·
Binder
·
Pigment
·
Filler/ Extender
·
Volatile organic compound (Thinner)
·
Driers
·
Additives (Anti skinning agents,
Anti settling agent, Plasticizers, fire retardants etc
Binder
Binders are usually resins or oils but can be inorganic compounds.
Binder is the actual film forming component & absolutely required
ingredient of any paint. It consists of a resin and a solvent thinner. It is
the part which solidifies to form the dry paint film when the solvent
evaporates. They are non-volatile & mainly polymers of various types. Different
resins form dry film on the substrate in different manners.
Function of binder
·
Binds pigment, fillers &
additives together
·
Imparts adhesion & strongly
·
It influences gloss, durability,
flexibility and toughness
Pigments
Pigments are finely ground inorganic or organic powders of higher
refractive index (> 1.5). Higher the RI, more the light is bent &
greater the opacity. Good opacity has good lighting absorbing & /or
scattering properties. The colour of pigment depends on the composition of the
pigment used. For example, White lead and titanium oxide is are white pigments
whereas red lead, ferric oxide, etc are red pigments. Good pigments are opaque,
non toxic and chemically inert.
Function of pigment
·
It provides opacity and colour to
paint ilm.
·
Give strength to the paint film.
·
Provides protection to paint by
reflecting harmful ultraviolet light.
·
Increases weather resistance of
paint film
Extender / Filler
Non-expensive commonly natural inorganic materials added to the paint
in order to increase its volume. Extenders are mainly inorganic substances
& do not provide colour to the paint but added to improve adhesion, ease of
sanding and film strength. As they are cheap in comparison to prime pigments,
they reduce overall cost of the paints.
Function
·
thickens the film
·
Increases volume, paint film
thickness
·
reduces cost of the paint
·
imparts toughness, abrasion
resistance & texture
·
Control consistency
Widely used fillers: Calcium carbonate, Gypsum, Ground silica, Barytes, Slate powder, French
chalk, china clay, asbestos, silica, mica, whiting etc.
Driers
Depending upon the nature of the solvent and film thickness, the
drying process may take as long as several hours. Thicker the film, longer the
drying time. If the drying process is artificially accelerated, there may be
problems with adhesion between the protective film and the metal surface.
Metallic salts of Lead, Manganese, Cobalt, etc. of organic acids are generally
used as drier.
Purpose
·
to accelerate the drying process.
Examples: Lead acetate,
Cobalt octate, Manganese octate, Litharge, Red lead, Lead octate, Manganese
dioxide, Zinc sulphate, etc.
Additives
Additives are small amounts of different chemical substances
improving or modifying the paint properties. Added to a paint in amounts 0.001%
& ≤ 5% & have a profound influence on physical & chemical
properties of the paint.
Function
·
Prevent clustering of pigments
·
Texturizers impart textures to the
coatings.
·
Antifreezers helps to withstand
exposure
·
Pigment stabilizers improve pigment
stability
·
Fire retardant properties
Methods of application of paints
Paint is applied to the surface through several methods. Some of
them are discussed below:
Roller coating: Roller coating method is used when the material to be painted is
in form of flat sheets. Roller is dipped in paint and rolled over the material
which needs to be painted. During the passage, the articles are painted
uniformly.
Brushing: An easy method of
applying paint is brushing. Brush is dipped in paint and then the brush is
applied on the surface. A non-viscous and covering paint is must to apply it on
material. A good brush should have flexible yet rigid bristles. Brush gives a
smooth finish on the surface. Paints who are more viscous are first mixed with
a thinner before getting applied.
Spraying: This method involves the application of paint using spray gun.
Spraying method is very useful for uneven surfaces. The process is quick but
more paint is required as compared to other methods. The spray can be directed
to the surface where fine molten droplets rapidly solidify and form the
coating. In recent advancements, paint is made of negative charge and the
article to be painted is made positive. So, when paint is sprayed, negatively
charged particles of paint is attracted to positively charged articles and
hence less paint is wasted.
Dipping: Dipping is the
simplest method of application of paint. The article to be painted is simply dipped
in paint and then the article is taken out and paint is dried. The major
disadvantage of dipping is that thickness of applied paint is not uniform.
Tumbling: Tumble spray
involves placing components in a specially designed, hexagonal shaped unit. A
controlled spray of atomized coating is then applied to parts as the unit
rotates. The hexagon design of the unit is important as the components are
flipped and rotated more than a simple cylindrical unit could. Once the coating
is applied, parts are loaded in baskets and baked in a batch oven.
Chemical resistant paints
Chemical resistant coatings are designed for metal, concrete
floors & walls, Wood & Fiber to prevent the abrasion from harsh
chemicals including the acids and several other abrasive materials. Chemical
resistant paints protect different surfaces from chemical. The chemical
resistance of a coating will depend on number of criteria such as
·
The type of chemical used and its
concentration
·
The duration for which the article
has been exposed to paint
·
Chemical resistant paints are
required in nuclear facilities, liquid spillages, storage of high temperature
chemicals, where enhanced lubrication is required, etc.
Heat resistant paints
Heat resistant paints are designed to withstand high working
temperature on commercial and industrial metallic surfaces and structures from
heat treatment applications. For example, chimneys of furnaces, incinerators operate
at very high temperature. This type of surface requires a paint which does not
breakdown at high temperatures. Heat resistant paint does not deliver fire
retardant properties but still can be used in industrial applications.
Generally high resistant paints are applied using spraying.
Cellulose Paint
Cellulose paints are highly toxic, flammable and very fast drying.
It is one of the industrial paints. Its raw material consists of the reaction
of cellulose obtained from cotton and wood with alcohol and acids.
Cellulose-based paints are not widely used in construction compared to
synthetic and water-based paints. The reason for this is that the harmful
chemicals it contains and the thinner used in thinning is cellulose-based.
Luminous Paint
Luminous paint or luminescent paint is paint that exhibits
luminescence. In other words, it gives off visible light through fluorescence,
phosphorescence, or radioluminescence. There are three types of luminous
paints: fluorescent paint, phosphorescent paint and radio luminescent paint.
Fluorescent paint
Fluorescent paints 'glow' when exposed to short-wave ultraviolet
(UV) radiation. These UV wavelengths are found in sunlight and many artificial
lights, but the paint requires a special black light to view so these
glowing-paint applications are called 'black-light effects'. Fluorescent paint
is available in a wide range of colours and is used in theatrical lighting and
effects, posters, and as entertainment for children.
Phosphorescent paint
Phosphorescent paint is commonly called
"glow-in-the-dark" paint. It is made from phosphors such as
silver-activated zinc sulphide or doped strontium aluminate, and typically
glows a pale green to greenish-blue color. The mechanism for producing light is
similar to that of fluorescent paint, but the emission of visible light
persists long after it has been exposed to light. Phosphorescent paints have a
sustained glow which lasts for up to 12 hours after exposure to light, fading
over time.
Radio luminescent paint
Radio luminescent paint is a self-luminous paint that consists of
a small amount of a radioactive isotope (radionuclide) mixed with a radio
luminescent phosphor chemical. The radioisotope continually decays, emitting
radiation particles which strike molecules of the phosphor, exciting them to
emit visible light.
Emulsion paint
Emulsion paint is water-based paint, that consists of pigment,
emulsifier, coagulant, and water. It's called emulsion because the pigments are
dispersed in water emulsified with an emulsifying agent. Emulsion paints are
quite popular for their durability, easy-to-use features, and low cost.
Emulsion paint consists of tiny polymer particles within which the pigments are
trapped. The particles are suspended in water, then as the paint dries the
particles fuse together creating a film of paint on the wall. Once this happens
the polymer can’t be resuspended in water, which is why you can’t wash a
water-based emulsion paint back off the wall once it has dried. Emulsion paints
are free from hazard and have no objectionable odour.
Metal Paints
Metal paints are paints which easily adhere to metal surfaces.
Paint coating on metal surfaces is applied on bodies for protection and
decoration. The coating on metal may be of galvanic type or barrier type.
Cement paint
Cement paints are prepared by mixing white cement with colouring
pigments, hydrated lime and fine sand. Cement paints are available in form of
powder of particular colour. The dispersion medium may be water or oil
depending on the purpose of coating. Before applying cement paint, a primer
coat consisting of dilute solution of sodium silicate and zinc sulphate is
necessary. Cement paints have water proofing capacity and give a stable and
decorative film.
Water paints or distempers
Distemper paint is an ancient type of paint made of water, chalk,
and pigment. It is bound with either an animal glue or the adhesive qualities
of casein, a resin that comes from solidified milk. The basic constituent of
Distemper Paint is chalk, lime, and water. Such kind of paint can be applied
directly on cement walls without any other coating on them without using the
primer. They are a cheaper option and they stay good for more than 3 to 4
years.
Varnishes
The Varnish is a transparent, hard, protective finish or film
primarily used in wood finishing and also for other materials. Varnish is a
homogeneous colloidal solution of natural or synthetic resins in oils or
thinners or both. It enhances and gives comfort to the grain of the wood and is
resistant to impact, heat, erosion, water, and alcohol. It can be used as a top
coat over a painted surface.
Characteristics of good varnish
·
It should be dry quickly
·
On drying it should form a hard,
tough and durable film.
·
It should have good weathering
properties, resist abrasion and wear well.
·
It should be able to retain its
colour and shine.
·
It should be uniform and pleasant
looking on drying.
Constituent of varnish
The ingredients of varnish are:
Resins
Solvents
Driers
Resins
Commonly used resins are copal mastic, amber gum and lac. Quantity
of varnish depends much upon the quality of resin used. Copal is considered to
be the best, toughest, hardest and is very durable for external work.
Solvents
These must suit the resins used. Boiled linseed oil is used to
dissolve copal or amber, turpentine oil for common resin or mastic, methylated
spirit for lac. Wood naphtha, because of its offensive smell is not suited for
superior works and is used only for cheap varnish.
Driers
These should be added only in small quantities as an excessive
injures varnish and impairs its durability. Litharge or lead acetate are the
commonly used driers in varnish added to accelerate drying process.
Different kinds of Varnishes:
Based on the different solvents used, varnishes are classified
under the following categories:
Oil Varnish
These are made by dissolving hard resins like amber or copal in
oil. They are slow to dry but are hardest and most durable of all varnishes.
They are suited for being used on exposed surfaces requiring polishing or
frequent cleaning and for superior works.
Turpentine Varnish
These are made from soft resins like mastic, common resin is
dissolved in turpentine oil.
Spirit Varnish
Varnishes in which spirit is used as a solvent is known as
spirited varnish or French Polish. Shellac is dissolved in spirit and the
product is applied in a thin layer. This varnish gives a transparent finish
thus showing the grains of the timber. These however, do not weather well and
as such are used for polishing wood work not exposed to weather.
Water Varnish
They consist of lac dissolved in hot water with borax, ammonia,
potash or soda just enough to dissolve the lac. Varnish so made withstands
washing. It is used for painting wall paper and for delicate work.
Japans
Japans are pigmented varnishes which are added to paint to give it
a good colour and lustre. Japans are generally used to paint bicycles and
electrical devices. Japans are usually of two types: Printer’s Japan and
decorative Japans. Printer’s Japans consists of resin dissolved in drying oil
containing drier and thinner. Printer’s Japans make paint more lusturous.
Decorative Japan is prepared by heating linseed oil and lead oxide. This
results in a solid mass which is known as lead oil. This lead oil is then mixed
with asphaltum and thinner to get decorative Japans.
Enamels
Enamel is a type of paint which is traditionally oil-based,
although variations like water-based or latex-based enamels are readily
available in the market. Enamel gets air-dried hard and used as protective as
well. Enamel is considered best for the materials which are of regular use and
thus enamel helps to protect the wear and tear products or the things that are
subject to remain outside the shelter and faces every type of climatic conditions
for example cars. Thus, enamel is considered best for materials like furniture,
vehicles or any kind of wood or metal.
Constituents of enamel
Pigments: Generally,
titanium dioxide and calcium sulphate is used as pigments for enamels. Pigments
as generally white. But when coloured pigments are used, they are referred to
as Japans.
Vehicles: Vehicles make
enamels of glossy finish. The vehicle of enamel is either oil and resin or only
resin. Vehicles increase the viscosity, gloss and colour of enamel.
Driers: Commonly used
driers are naphthenates or resonates of copper, zinc, etc.
Thinners: Thinners used are
turpentine, acetone, etc.
Lacquers
Lacquer is a type of hard and usually shiny coating or finish
applied to materials such as wood or metal. It is most often made from resin
extracted from trees and waxes. Lacquer is a substance that provides a strong
and shiny finish. The main objective of using lacquer is to provide a highly
shiny and glossy coat on the surface of the wood or any object that is being
coated. Lacquer is not a durable substance, and it is only used for its
appearance.
Constituents of liquor
Cellulose derivative: Cellulose derivatives give
durability and water resistance property to the film.
Resins: Resins are used to
provide adhesion, thickness and gloss to film. Resins generally used in
lacquers are copal, alkyd, etc.
Solvents: The solvent is
used to dissolve the film forming material. The solvents used are esters,
ketones, alcohols, etc.
Plasticizers: They reduce
the brittleness of liquor. This improves the adherence and durability of
plasticizers. Commonly used plasticizers are castor oil, chlorinated diphenyls,
etc.
Diluent: Diluents are
generally coal-tar products which reduces the viscosity as well as cost of the
liquor. Benzol and naptha are used as diluents.
Difference between enamel and lacquer
|
Parameters of Comparison
|
Lacquer
|
Enamel
|
|
Definition
|
Lacquer is a coating solution that is
primarily used to give a shiny coating to surfaces
|
Enamel is a hard paint solution that is used
for coloring as well as to give a hard coating on the surface
|
|
Durability
|
Lacquer is not durable and long-lasting
|
Enamel is a durable and long-lasting coating
|
|
Drying time
|
Lacquer dries very quickly, right after
application
|
Enamel takes a longer time to dry
|
|
Thinner
|
Specific lacquer thinners are used
|
Spirits are used as thinner agents while
applying enamel
|
|
Demerit
|
Lacquer develops bubbles if not applied
properly
|
Enamel does not have such bubble formation
|