Water
Water is essential
to life. Without it, the biosphere that exists on the surface of the earth
would not be possible. It is also the only known chemical compound that occurs
in all the three physical states viz., solid (snow, hail, sleet and ice),
liquid (rain, water droplets) and gas (water vapors). Water is the chemical
substance with chemical formula H2O, one molecule of water has two
hydrogen atoms covalently bonded to a single oxygen atom.
Water covers more
than 70 % of the earth’s surface, of which 97% is in the ocean, which is unfit
for human consumption and other uses because of its high salt content. Of the
remaining three percent, 2% is locked in the polar ice caps and glaciers and
only one percent is available as fresh water in rivers, lakes, streams,
reservoirs, and ground water which is suitable for human consumption.
A continuous and supply of clean water is essential for the survival and health
of all living organisms.
Characteristic of
water
Physical
characteristics of water
Turbidity
of water: Turbidity is the amount of
suspended matter in water and expressed in parts per million
Color: The colour of a good water is transparent. The colour of water is
measured by tintometer.
Taste and
odour: Preferably, the water should be free from taste
and odour.
Chemical
characteristics of water
Total
solids and suspended solids: The suspended
solid can be found by filtering the water sample. Total permissible amount of
solids in water is generally limited to 500 ppm.
pH of
water: pH of water should be 7.
Hardness
of water: Hard waters
are undesirable because they may lead to greater soap consumption, scaling of
boilers, causing corrosion and incrustation of pipes, making food tasteless
etc.
Sources of water
Source water refers
to bodies of water (such as rivers, streams, lakes, reservoirs, springs, and
ground water) that provide water to public drinking-water supplies and private
wells. Water on the earth is broadly classified under two categories. They are
Surface water
Ground water
Surface
water
Surface water is
quite a broad term when we look at it. It consists of any above-ground water
which gets collected. For instance, we have ponds, rivers, lakes, oceans and
more. Surface water collects on the ground or in a stream, river, lake,
reservoir, or ocean. Surface water is constantly evaporating out of water
bodies, seeping into ground water supplies, and being replenished by rain and
snow.
Surface water is
the most used source of water. It accounts to at least 80 per cent of the water
used by living beings. Following are the sources of surface water
Rain
water: Rain water is the purest form. It is
obtained by natural process of evaporation and condensation of water.
River
water: A river is a
ribbon-like body of water that flows downhill from the force of gravity. A
river can be wide and deep, or shallow enough for a person to wade across. A
flowing body of water that is smaller than a river is called a stream, creek,
or brook. Some rivers flow year-round, while others flow only during certain
seasons or when there has been a lot of rain. The largest rivers can be
thousands of miles long.
Lake: A lake is a body of water that is surrounded by land. There are
millions of lakes in the world. They are found on every continent and in every
kind of environment—in mountains and deserts, on plains, and near seashores.
Lakes vary greatly in size. Some measure only a few square meters and are small
enough to fit in your backyard. Such small lakes are often referred to as
ponds. Other lakes are so big that they are called seas. The Caspian Sea, in
Europe and Asia, is the world’s largest lake.
Sea water: This is the most impure form of
natural water. Rivers throw all the impurities carried with water into sea. The
continuous evaporation of water from surface of sea increases concentration of
dissolved impurities. This sea water contains about 3.5% of dissolved
impurities, out of which about 2.5% is sodium chloride. Thus, sea water cannot be
used directly for domestic or industrial purpose.
Ground water
When we say
groundwater, we mean the source of water which is found beneath the layer of soil.
It exists in the soil and between rocks and other things. Groundwater
contributes to 30% of water which we use in our daily lives.
A part of rainwater
which reaches earth’s surface or water from river percolates into the earth.
This water travels downwards, during which it comes in contact with various
mineral salts present in the soil and dissolves some of them. Water continues
its downward flow till it meets hard rock.
Spring and well
water is generally clear in appearance as it is filtered through soil layers,
but contains considerable quantity of dissolved salts. The water from well and
spring contains more hardness. The underground water is suitable for domestic
use.
Impurities of
water
Water available on
earth is not in its purest form. The water is generally contaminated with
materials which are often referred to as impurities. The common impurities
present in natural water may be classified as follows:
Suspended particles
Dissolved
impurities
Colloidal
impurities
Biological
impurities
Suspended
impurities: Suspended impurities are the
impurities that are partially soluble in water, and kept suspended in water.
These impurities consist of clay, mud, algae, organic matter, etc. Such
impurities are referred to as suspended impurities. They are present in most of
the surface water. These impurities are generally visible with naked eyes.
Suspended impurities in water if consumed can result in a range of medical
ailments and illnesses.
Dissolved
impurities: The
dissolved impurities include salts and minerals. These minerals are not
important and are harmful to organisms. Dissolved impurities may also include
organic and inorganic salts as well as gases.
Gases: The dissolved gases present in water
may include oxygen, carbon dioxide, nitrogen, etc. These gases are soluble in
natural water.
Mineral
salts: The commonly
observed salts in natural water are carbonate, bicarbonates, magnesium, etc.
When salts of calcium and magnesium are present in water, it becomes hard
water.
Colloidal
impurities: Colloidal
impurities are such impurities which doesn’t settle down even in standing water
and cannot be removed by filtration. Size of colloidal particle is about 10–4 cm to 10–7 cm.
Biological
impurities: Biological
impurities in water are caused by the presence of living organisms. These
include algae, protozoa, pathogens, bacteria, viruses, microbes, and parasites
along with their cysts (eggs) in contaminated water. Such water is dangerous
for human consumption.
Hard and soft
water
Soft water
Soft water is the
type of water that contains lower amounts or lower concentrations of minerals
like calcium and magnesium. However, soft water may include salt like sodium or
potassium dissolved in it. Soft water tastes salty while drinking.
Hard water
The water with
naturally present minerals like magnesium and calcium with detectable amount is
called hard water. Hard water contains more minerals, but that doesn’t mean
it’s contaminated. Contamination is different than mineral content and refers
to germs and bacteria instead. Minerals are valuable nutrients for your body,
although too many may pose health risks. Minerals improve water’s taste.
Difference
between hard water and soft water
|
Hard Water
|
Soft Water
|
|
It is rich in minerals
|
Contains very few elements
|
|
Soap is not so effective
|
Soap is easily effective
|
|
No foam and lather from soaps
|
Bubbly lather from soaps
|
|
Leaves spots on the washed dishes after they are dried
|
Does not leave any spots on dishes after they are dried
|
|
Contains minerals like magnesium and calcium
|
Contains sodium ion
|
|
Sometimes preferred drinking water
|
Sometimes not preferred drinking water
|
|
Example: Groundwater like deep wells
|
Example: Rainwater
|
|
Hair and skin become dry
|
Hair and skin become soft
|
Causes of
hardness of water
Hardness of water
is due to the presence of calcium and bicarbonates present in it. Deposition of
calcium and magnesium salts make the water hard. Water receives such mineral
while moving in its examples. Some reasons are mentioned below:
Rainwater absorbs
carbon dioxide from air and also from decaying plants on soil. This rain water
when flows over the rocks containing calcium and magnesium carbonate reacts
slowly with the substances forming bicarbonates. Chloride and sulphate of
calcium and magnesium are also present on the surface layer. These salts are
soluble in water. Thus, water becomes hard.
Types of
hardness
There are two types
of hardness. They are as follows
·
Temporary or carbonate hardness
·
Permanent aur non carbonate hardness
Temporary
or carbonate hardness
The presence of
magnesium and calcium carbonates in water makes it temporarily hard. In this
case, the hardness in water can be removed by boiling the water.
When we boil water
the soluble salts of Mg(HCO3)2 is converted to Mg(OH)2
which is insoluble and hence gets precipitated and is removed. After
filtration, the water we get is soft water.

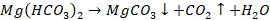
Permanent
or non-carbonate hardness
Permanent or
non-carbonate hardness is due to the presence of dissolved chlorides, sulphates
of calcium and magnesium. Permanent hardness cannot be destroyed on boiling. It
requires special chemical treatment for the removal of hardness. There are
several softening methods that is used to treat permanent hard water. Some of
them
Difference
between temporary and permanent hardness of water
|
Temporary hardness
|
Permanent hardness
|
|
This type of hardness is due to soluble bicarbonates of calcium
and magnesium.
|
It is due to soluble chloride and sulphate salts of calcium and
magnesium.
|
|
Since it is due to carbonates, and hence it is known as
carbonate hardness.
|
It is due to salts other than carbonates and hence known as non-
carbonate hardness
|
|
Temporary hardness can be removed by boiling.
|
Permanent hardness cannot be removed by boiling. They require
different softening techniques
|
Harmful
Effects of Hard Water
·
Some of the most common signs of hard water include:
·
Linens and clothes look dull and feel rough.
·
Ugly stains on white porcelain and scale build-up on faucets
·
Low water pressure from showers due to clogged pipes.
·
Chalky, white residue or spots appear on dishes.
·
Strains appearing in the shower.
Degree of
hardness
The amount of
hardness causing substances in a certain volume of water measures the degree of
hardness. The hardness of water is always calculated in terms of calcium
carbonate CaCO3. However, it is never responsible for causing hardness
as it is insoluble in water.
The choice of CaCO3
is due to its molecular weight 100 and equivalent weight 50. The
units commonly used to express hardness of water is as follow
Different
units used to express degree of hardness
·
ppm (parts per million) = This is weight in milligrams of CaCO3 equivalent to
all hardness causing substance in one million (106) milligrams of
water.
·
mg/L (milligram per litre) = weight in milligrams of CaCO3 equivalents in on
litre of water.
·
⁰Cl (degree
Clark) = weight in grams
of CaCO3 equivalents in 70,000 grams of water.
·
⁰Fr (degree
French) = weight in
grams of CaCO3 equivalents in 105 grams of water.
Relationship
between these units
1 ppm = 1 mg/L = 0.1⁰Fr = 0.07 ⁰Cl
Boiler and steam
generation
The manufacturing
industries need water for a great variety of purposes out of which steam
generation is the most important one. Hard water has an adverse effect on steam
boilers. Hence, water for raising steam in boilers must be soft and must not contain too much impurities. Impure water produces
foam, and also deals with several other problems such as corrosion, caustic
embrittlement, scale and sludge formation.
A boiler or steam generator is a device used to create steam by
applying heat energy to water. It is a closed vessel made of high-quality steel
in which steam is generated from water by the application of heat. The water
receives heat from the hot gases formed by burning fuel through the heating
surface of the boiler. Steam is mainly required for power generation, process
heating, and space heating purposes.
Problems associated with hard water in boiler
Corrosion: The most common problem associated with boiler due to the use of
hard water is corrosion. Corrosion causes the decaying of boiler. Hard water
consists of dissolved gases and salts which chemically reacts with metallic
surface of boiler. This leads to decaying of boiler.
Caustic embrittlement: Caustic embrittlement is also a type of corrosion for boiler.
This type of corrosion is caused by the use of highly alkaline water in boiler
and it is generally observed in the boiler which operates under a high
pressure. During water softening process, small amount of sodium carbonate is
added. In high pressure boilers, sodium carbonate decomposes to give sodium
hydroxide and carbon dioxide.

Due to the form of sodium hydroxide (caustic soda), water becomes
more alkaline. There are minute cracks in the inner lining of boiler. The
alkaline water flows into the cracks of boiler lining due to capillary action.
Water evaporates and dissolved caustic soda is left behind. The alkaline action
of caustic soda attacks the surrounding areas of cracks thereby dissolving iron
material of the boiler. This causes embrittlement of boiler parts,
particularly, at stressed parts like rivets, bends, joints, etc. causing even a
failure of the boiler.
Prevention for caustic embrittlement
·
Use sodium phosphate instead of
sodium carbonate for softening water.
·
Use lignin as additives to the
boiler water which will block the minute cracks thereby preventing infiltration
of caustic soda solution.
·
By adjusting the alkalinity of water
to optimum level.
Priming and Foaming: When a boiler is steaming
(i.e., producing steam) rapidly, some particles of the liquid water are carried
along-with the steam. This process of 'wet steam' formation is called priming. Priming
is caused by
·
the presence of a large amount of
dissolved solids
·
high steam velocities,
·
sudden boiling
·
improper boiler design
·
sudden increase in steam-production
rate.
Foaming is the production of persistent foam or bubbles in
boilers, which do not break easily. Foaming is due to presence of substances
like oils (which greatly reduce the surface tension of water).
Priming can be avoided by:
(i) fitting mechanical steam purifiers;
(ii) avoiding rapid changing steaming rate;
(iii) maintaining low water levels in boilers
(iv) efficient softening and filtration of the boiler-feed water.
Foaming can be avoided by:
(i) adding anti-foaming chemicals like castor oil
(ii) removing oil from boiler water by adding compounds like
sodium aluminates.
Limitation of priming and foaming
(i) dissolved salts in boiler water are carried by the wet steam
to super-heater and turbine blades, where they get deposited as water
evaporates. This deposit reduces their efficiency
(ii) dissolved salts may enter the parts of other machinery, where
steam is being used, thereby decreasing the life of the machinery;
(iii) actual height of the water column cannot be judged properly,
thereby making the maintenance of the boiler pressure becomes difficult.
Scale and sludge formation
In a boiler, water is continuously evaporated and converted into
steam. As a result, the water becomes saturated due to increase in the
concentration of dissolved impurities. Finally, a stage is reached where the
ionic products of these salts exceed their solubility product and are thrown
out as precipitates on the inner walls of the boiler.
Sludge: It is soft, loose
and slimy precipitate formed within the boiler. It is formed at comparatively
colder portions of the boiler and are collected at the bends. It is formed by
substances which have greater solubility in hot water than in cold water, e.g.,
MgCO3, MgCl2, CaCl2, MgSO4, etc
Disadvantages of sludge formation
·
Sludges are poor conductors of heat,
so they tend to waste a portion of heat generated.
·
Excessive sludge formation disturbs
the working of the boiler.
Sludge can be removed by using
1) Softened water
2) by blow down operation i.e., drawing off a portion of the
concentrated water.
Scales: It is hard
deposits firmly sticking to the inner walls of the boiler. The hardness of
scale depends upon the nature of impurities present in the water. The most
troublesome scales are formed due to the presence of sulphates and silicates of
calcium and magnesium. Such scales are non-porous and non-conductor of heat.
Scales are difficult to remove, even with the help of hammer & Chisel.
Scale is formed due to:
i) Decomposition of Calcium bicarbonate:

In high pressure boilers, CaCO3 is soluble due to
formation of Ca (OH)2

ii) Deposition of CaSO4
Solubility of CaSO4 decreases with increase in temperature.
It is completely insoluble in super-heated water.
Hard scale formation takes place in high pressure boilers
iii) Hydrolysis of Magnesium salts:
Soft scale formation due to hydrolysis of Mg salts in high
pressure boiler.
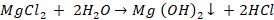
Disadvantages of scale formation:
i) Wastage of fuel:
Rate of heat transfer is greatly reduced due to poor conductivity
of scales
Overheating is required for steady supply of heat hence fuel
consumption increases.
ii) Lowering of boiler safety:
To supply steady heat overheating is required, which makes the
boiler material weak &soft. Results in distortion of boiler tube &
makes the boiler unsafe to bear the high pressure.
iii) Decrease in efficiency of boiler:
Deposition of scales in valves and condensers choke them
partially& decreases the efficiency of boiler
iv) Danger of Explosion:
Due to uneven expansion the thick scales get cracked, results in
formation of large steam & develops high pressure. It may cause explosion
of boiler.
Removal of scales:
·
Mechanical/chemical method
·
Loosely adhering scales are removed
with the help Scraper/wire brush
·
Brittle scales are removed by giving
Thermal shocks
·
Loosely adhering scales are removed
by frequent blow down operation (frequently removing precipitates)
Softening methods of water
The process of removing soluble calcium and magnesium salts from
hard water is called softening the water. During the process of softening of
hard water, the soluble salts are converted into insoluble salts. These
insoluble salts then can be filtered and soft water can be obtained. Several
methods are used for the softening of water. Some of them are as follows:
·
Boiling
·
Lime-soda process
·
Zeolite or permutit process
·
Ion exchange process
·
Clark’s method
Boiling
Boiling water only removes temporary hardness. Boiling water is
certainly one of the most effective ways to soften it. The boiling will have
the effect of draining the hard water minerals to the bottom, like calcium and
magnesium. Boiling precipitates the dissolved minerals out of the water. Since
boiling removes the water’s calcium content, the result is softer water.
When you boil water, the salts precipitate leaving clean, soft
water.
Put some water in a pot and leave it boiling for at least five
minutes for the best results. After the water has boiled sufficiently, turn the
heat off.
Let the water cool. Allow sufficient time, so the mineral deposits
sink to the bottom. Next, pour the soft water from the pot and leave the
mineral deposits in the pot.
Boiling is a quick and cheap way to fix hard water for consumption
purposes. However, it only addresses temporary hardness and not permanent
hardness.
Clark’s method
Clark’s method is one of the solutions for treating hard water by
adding slaked lime into the water. Hardness of water can be defined as the
dissolved content of divalent metal cations in water. In Clark’s method, hard
water is treated with Ca (OH)2. This method removes hardness by
conversion of bicarbonates into carbonates.
Clark's technique for softening water:
Hard water is softened with in Clark's water softening technique
(slaked lime). Clark's reagent is Calcium Hydroxide. Calcium hydroxide is used
in Clarke's method to soften water (lime). It eliminates transient hardness. By
turning bicarbonates into carbonate, it softens the water.
Slaked lime, either in solid or liquid form, is added to water
using this technique. Soluble bicarbonates are changed into insoluble
carbonates as a result.
In this method, a calculated amount of lime is added to hard
water, it precipitates out calcium carbonate and magnesium hydroxide which can
be filtered off.
Chemical reaction involved in Clark’s method
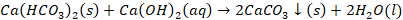
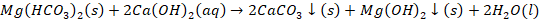
Lime soda method
The water is treated with lime or a combination of lime and soda
ash (carbonate ion). These chemicals react with the hardness and natural
alkalinity in the water to form insoluble compounds. The compounds precipitate
and are removed from the water by sedimentation and, usually, filtration. When
water has minimal magnesium hardness, only calcium needs to be removed. Only
enough lime and soda ash are added to water to raise pH to between 10.3 and
10.6, and calcium hardness will be removed from the water.
When lime and soda ash are added, hardness-causing minerals form
nearly insoluble precipitates. Calcium hardness is precipitated as calcium
carbonate CaCO3. Magnesium hardness is precipitated as magnesium
hydroxide Mg (OH)2. These precipitates are then removed by
conventional processes of coagulation/flocculation, sedimentation, and
filtration.
Chemical reaction involved in soda ash method
|
Hardness
|
Lime
|
Precipitate
|
|
 + +
|

|
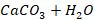
|
|
 + +
|

|

|
|
 + +
|

|

|
|
 + +
|

|

|
Soda ash method
Soda Ash is the common name for sodium carbonate, which is a
chemical compound with the formula Na2CO3. This compound
is also known as washing soda and soda crystals. In this method water is
treated with a calculated amount of soda ash (also referred to as washing
soda). Soda ash converts the chlorides and sulphates of calcium and magnesium
into their respective carbonates which get precipitated.
Chemical reaction involved in soda ash method
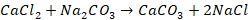
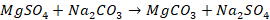
Zeolite or permutit process
The zeolite softening process is used for removing both the
temporary and permanent hardness of the water by precipitating the calcium and
magnesium present in water as insoluble zeolites.
In this process of softening of water, there is an exchange of Ca2+
and Mg2+ ion with the help of zeolite. Hence it is called the
zeolite softening process. Zeolite is micro porous mineral which is used as
catalyst in many industrial purposes such as water purification. The zeolites
are hydrated aluminosilicates and general composition is Na2Al2Si2O8.
Zeolites are of two types i.e., synthetic and artificial. The natural zeolite
that is used for water softening is gluconites. Permutit is the synthetic
zeolite that is most used in water softening. Permutit are more porous, glassy
and have higher softening capacity than gluconites.

Zeolite process is commercially accepted for the fact that zeolite
can be easily regenerated. When Ca2+ and Mg2+ ions
containing hard water passes through a bed of sodium zeolite, the sodium ions
are replaced by the calcium and magnesium ions. When all sodium ions are
replaced by calcium and magnesium ions, the zeolite becomes inactive. Then
zeolites need to be regenerated. Brine solutions are passing through the bed of
inactivated zeolite. The following reactions takes place to form Na2Ze.


The chemical reactions that take place during softening process
are as follows:

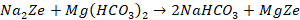

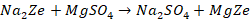
Ion exchange method for water softening

Aside from industry, ion-exchange softeners are widely used in
small water systems and individual homes. Ion-exchange resins, exchanges one ion
from the treated water for another ion in the resin (sodium is one component of
softening salt, with chlorine being the other). Sodium is exchanged for calcium
and magnesium by ion exchange resin. Certain organic compounds possess a
property like zeolite i.e., they are capable of exchanging ions. Such organic synthetic compounds are known as resins. There
are two types of resins
Cation exchange resins: These resins are capable of exchanging
rapidly cations by H+ ions. Cation exchange resins can be
represented as RH2, so their exchange reaction with cations is

Anion exchange resins: These resins are capable of exchanging
rapidly anions by OH– ions. Anion exchange resins can be
represented as

If hard water is passed first
through cation exchanger and then through anion exchanger, the resulting water
will be free from both cations and anions and water is said to be deionised or
demineralised.
The process of ion exchange is
carried out as follows:
It consists of two cylindrical towers, out of which the first
tower consists of cation exchanger (RH2) and the other one consists
of anion exchanger[R’(OH)2]. Hard water is first allowed to pass
through a tower containing cation exchanger, which removes all the cations like
Ca++, Mg++, Na++ and releases H+ ions



Thus, the anions like chlorides, sulphates and bicarbonates are
converted into their corresponding acids HCl, H2SO4 and H2CO3.
In other words. This acidic water is then passed through another tower
containing an ion exchanger, where acids are converted into water.
Consequently, the water thus produced is free from all ions and is virtually
distilled water. The water is finally freed from dissolved gases like CO2
by passing it through a degasifier, which is a tower whose sides are connected
to vacuum pump. High temperature and low pressure reduce the quantity of
dissolved CO2 and O2 in water.
Difference between ion-exchange process and zeolite process
|
Ion-exchange process
|
Zeolite process
|
|
This process can produce softened water with residual hardness
ranging between 0 to 2 ppm.
|
This process can produce softened water with residual hardness
ranging between 0 to 15 ppm.
|
|
The resultant water is suitable for all types of boilers,
especially high pressure boilers.
|
The resultant water is not suitable for use in high pressure
boilers. Water can be used only in low or medium pressure boilers.
|
|
Capital cost is higher
|
Capital cost is less
|
|
It occupies more space
|
It occupies less space.
|
|
This process is useful for acidic as well as alkaline water.
|
This process is not useful for highly acidic water.
|
Plumbo solvency
All drinking water is plumbosolvent, which means it can dissolve
very small amounts of metals if they come into contact with them. Plumbo
solvency refers to the dissolution of lead in water. It increases lead
concentration in water that passes through lead pipes. This makes water unfit
for human consumption and may lead to health hazards. Lead is toxic. It affects
the functioning of heart, kidneys, reproductive and nervous system.
Water plumbosolvency can be counteracted by reaching a pH of 7.5
by increasing the pH with lime or sodium hydroxide (lye) or by providing
protection on the inside of lead pipes by applying phosphate to water treatment
works.
While the optimal pH for plumbosolvency prevention is 7.5,
performance in the range pH 7.2-7.6 is still very good. Attaining this pH has
been shown to decrease blood lead concentrations in the population.
Additionally, chlorinating water eliminates dissolved lead. With
this purpose, if the water could have been in contact with lead, water to be
used for drinking or cooking food can never be drawn from a hot-water tank.
Water should be taken from a cold water tap and heated in a saucepan or kettle
without lead or lead solder.
Water treatment methods
Following methods are generally carried out to make water fit for
drinking
·
Sedimentation
·
Coagulation
·
Filtration
·
Sterilization
Sedimentation
Sedimentation is the process of removing suspended impurities by
allowing the water to stay undisturbed for some period of time in large tanks
when most of the suspended particles settle down due to the force of gravity.
The clear water is then taken out from the tank with the help of pumps.
Coagulation
Coagulation is the chemical water treatment process used to remove
solids from water, by manipulating electrostatic charges of particles suspended
in water. This process introduces small, highly charged molecules into water to
destabilize the charges on particles, colloids, or oily materials in
suspension. Selecting the right coagulant for a system will enhance overall
system performance, and particularly improve solids removal efficiency by
enhancing filter and clarifier performance. The commonly used coagulants are
the salts of iron and aluminium. These coagulates react with bicarbonates
present in water and form bulky gelatinous precipitate called flock. These
flocks absorb or catch suspended fine particles from water and form bigger
flocks, which settle down quickly. The addition of coagulants to water also
removes colour, odour and improves its taste.


Filtration
Filtration is a process of removing insoluble colloidal and
bacterial impurities by passing water through a bed of proper sized material.
During filtration, the clear water passes through filters that have different
pore sizes and are made of different materials (such as sand, gravel, and
charcoal). These filters remove dissolved particles and germs, such as dust,
chemicals, parasites, bacteria, and viruses. Activated carbon filters also
remove any bad odours.
Water treatment plants can use a process called ultrafiltration in
addition to or instead of traditional filtration. During ultrafiltration, the
water goes through a filter membrane with very small pores. This filter only
lets through water and other small molecules (such as salts and tiny, charged
molecules).
Sterilization
Sterilisation (also referred to as disinfection in other sources)
is the final process to make potable water. It is where all microorganisms are
killed or removed. The process of destroying these disease-causing bacteria and
microorganisms etc from water is known as disinfection or sterilization of
water. The chemicals used for sterilization are known as sterilizers. The
disinfection of water can be carried out by any of the following methods
·
Boiling
·
Chlorination
·
Ozonisation
·
Aeration
·
Ultra-violet rays
Boiling: This is the simplest
method of sterilization. More often, sterilization is the boiling of water
before domestic use. Boiling kill all the pathogenic bacteria like cholera and
typhoid within five minutes. But this method is useful only for household
purposes, because this process is quite expensive for industrial processes.
Chlorination: The
chlorination can be carried by either of the following (i) By using chlorine
gas (ii) By adding bleaching powder (iii)
By using chlorine gas Cl2
Chlorination is the process of adding chlorine to drinking water
to kill parasites, bacteria, and viruses. Different processes can be used to
achieve safe levels of chlorine in drinking water. Using or drinking water with
small amounts of chlorine does not cause harmful health effects and provides
protection against waterborne disease outbreaks. It reacts with water to form
hypochlorous acid and nascent oxygen; both are germicides.
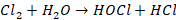
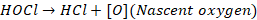

However, excess of chlorine should be avoided because it produces
unpleasant odour, taste and irritating effect on mucous membrane. The treated
water should not contain more than 0.1-0.2 ppm of free chlorine.
By adding bleaching powder (CaOCl2)
Bleaching powder is a good sterilizer for small water works. Bleaching
powder which is chemically called as calcium oxychloride (CaOCl2) is
an active agent which is used to kill germs and bacteria in drinking water. The
main content of the bleaching powder is the Chlorine element which acts as the
main disinfectant material.
Bleaching powder when exposed to moisture will release chlorine and
this chlorine kills off the germs and disinfects the area. If this is added to
water, the chlorine released will react with water and will cause production of
oxygen in the atomic state which is highly reactive.

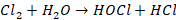
 Disadvantages of using bleaching powder
Disadvantages of using bleaching powder
·
Bleaching powder introduce calcium
in water thereby making it more hard.
·
If used in an excess amount, it
imparts a bad taste and disagreeable smell to water.
By using chloramine (ClNH2)
Chloramination is the process of adding chloramine to drinking
water to disinfect it and kill germs. It is sometimes used as an alternative to
chlorination. Chloramines are a group of chemical compounds that contain
chlorine and ammonia. The particular type of chloramine used in drinking water
disinfection is called monochloramine which is mixed into water at levels that
kill germs but are still safe to drink. Chlorine and ammonia are mixed in ratio
2:1 by volume to produce a compound known as chloramine.
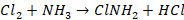


Ozonisation
This is an effective method of sterilization of water. Ozone O3
is unstable and it decomposes into molecular oxygen O2 and nascent
oxygen O


The nascent oxygen thus produced is very effective for killing all
the germs and bacteria. In ozonisation, water is allowed to percolate through a
tower having perforated partition. Ozone is allowed to enter from the bottom
which kills the germs when they come in contact with water. Sterilized water is
collected at the bottom of the tank. This method is quite expensive and hence
not commercially accepted.
Aeration
Aeration treatment consists of passing large amounts of air
through water and then venting the air outside. The air causes the dissolved
gases or volatile compounds to release from the water. The air and the
contaminants released from the water are vented. This is the most modern method
of purifying water for town supply. Water is forced under pressure through a
perforated pipe. As water sprays into air, it comes in contacts with the oxygen
of air and it is exposed to the ultra-violet rays of the sun. This kills the
bacteria and the oxygen oxidises organic matter present in the water. It
removes colour and odour also. Natural aeration takes place in streams and
rivers when the water flows slowly in its bed or when it falls from a certain
height.
Ultra violet rays
The invisible ultra violet rays are very effective in killing all
types of bacteria. This method is widely used for disinfection of swimming pool
water, because it does not require any chemical to be mixed with water. This
method requires focussing of uv rays of water which makes this method quite
expensive. Hence this method is not employed for municipal water supply.