Metals and Alloys
Metallurgy
Metallurgy is defined as a
process that is used for the extraction of metals in their pure form. It is a
process of extraction of metals from their ores economically and profitably.
Metals are commercially extracted from minerals at low cost and minimum effort.
Metallurgy deals with the process of purification of metals and the formation
of alloys.
Minerals
Minerals are substances
naturally formed in the earth. A mineral is a naturally occurring inorganic
substance with a definite chemical composition and ordered atomic structure. Minerals
are native forms in which metals exist. Metals are commercially extracted from
minerals at low cost and minimum effort. Minerals are broadly classified as
Primary
minerals: Minerals which were formed by igneous process
that is from the cooling down of the molten materials called magma are referred
to as primary minerals. For example, quartz, mica, etc.
Secondary
minerals: Secondary minerals are
formed with the help of primary minerals. Primary minerals are altered to form
secondary mineral. For example: mica is altered to form illite.
Ores
The metal ores are found in the
earth’s crust in varying abundance. The extraction of metals from ores is what
allows us to use the minerals in the ground. Ores are very different from the
finished metals that we see in buildings and bridges. An ore is a naturally
occurring deposit of geologic material (rock) that includes a sufficient
quantity of one or more valuable elements or compounds that it can be extracted
for economic gain. These valuable elements include metals like copper,
platinum, iron, lead, gold, silver, aluminum, nickel, and many more. Ores may
also contain nonmetals like silicon or diamond. These valuable substances can
be extracted and used in a variety of ways, from use in jewelry making to the
manufacturing of electronic devices. Ores vary widely depending on the chemical
composition of the parent rock, as well as the natural process by which they
were formed.
Difference between Ores and Minerals
Here we
have provided the major differences between Minerals and Ores.
|
Minerals
|
Ores
|
|
All the naturally occurring substances that are present in the
earth’s crust are known as Minerals.
|
Ores are usually used to extract metals economically. A large
number of ores are present.
|
|
All Minerals are not ores.
|
All ores are minerals.
|
|
Minerals are native forms in which metals exist.
|
Ores are mineral deposits.
|
Some ores and minerals
|
Iron
|
Haematite
Magnetite
Siderite
Iron pyrites
|
Fe2O3
Fe3O4
FeCO3
FeS2
|
|
Copper
|
Copper
pyrites
Malachite
Cuprite
Copper glance
|
CuFeS2
CuCO3.Cu(OH)2
Cu2O
Cu2S
|
|
Zinc
|
Zinc
blend/Sphalerite
Calamine
Zincite
|
ZnS
ZnCO3
ZnO
|
Gangue: Gangue
are impurities associated with minerals and ores. These impurities are removed
to extract metals
Flux: A
substance which is added to the charge in the furnace to remove the gangue
(impurities) is known as flux. Flux is a substance introduced in the smelting
of ores to promote fluidity and to remove objectionable impurities in the form
of slag. Limestone is commonly used for this purpose in smelting iron ores.
Other materials used as fluxes are silica, dolomite, lime, borax, and fluorite.
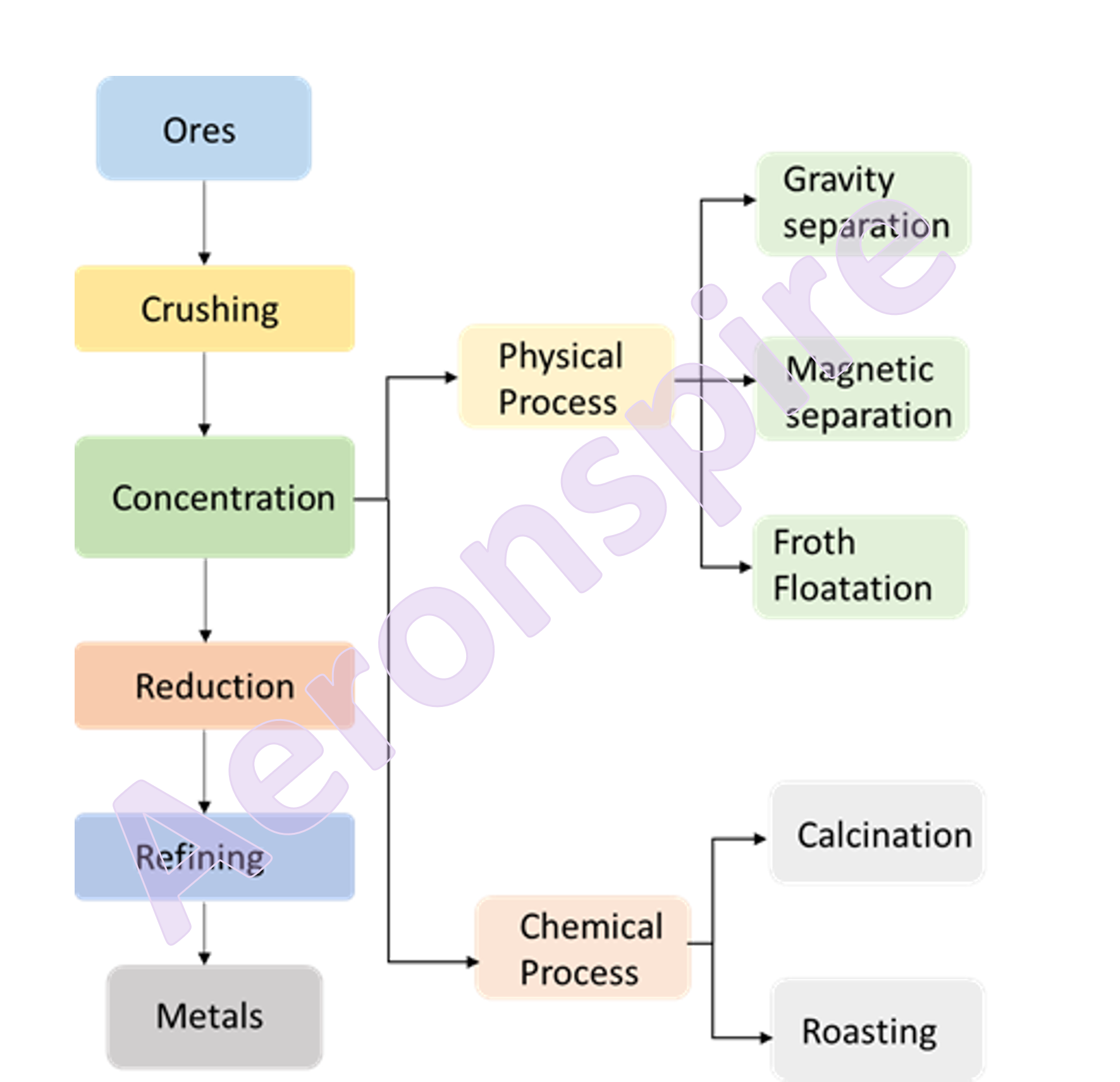
Steps involved in
metallurgical process
Generally following operations
are performed on ores to extract metals
Step 1: Crushing
Step 2: Concentration
Step 3: Reduction
Step 4: Refining
Crushing
The
ores occur in nature as huge lumps. These lumps are unsuitable for further
steps. They are broken to small pieces with the help of crushers or grinders.
These pieces are then reduced to fine powder with the help of a ball mill or
stamp mill. This process is called pulverisation. The amount of
crushing depends on the size of lumps. Different method of crushing is used for
different size of lumps.
Concentration
The
ores are usually found mixed up with large amounts of non-metallic impurities
such as, sand, mica, limestone, felspar, earthy and rocky impurities. These
unwanted impurities are called gangue or matrix and have to be removed before
extracting the metals. The process of removal of unwanted impurities
(gangue) from the ore is called ore concentration or ore dressing or ore
benefaction. The powdered ore is concentrated by one of the physical or
chemical process. The physical process involves gravity separation, magnetic
separation and froth floatation process. Chemical method involves calcination
and roasting.
Physical Process
Gravity separation
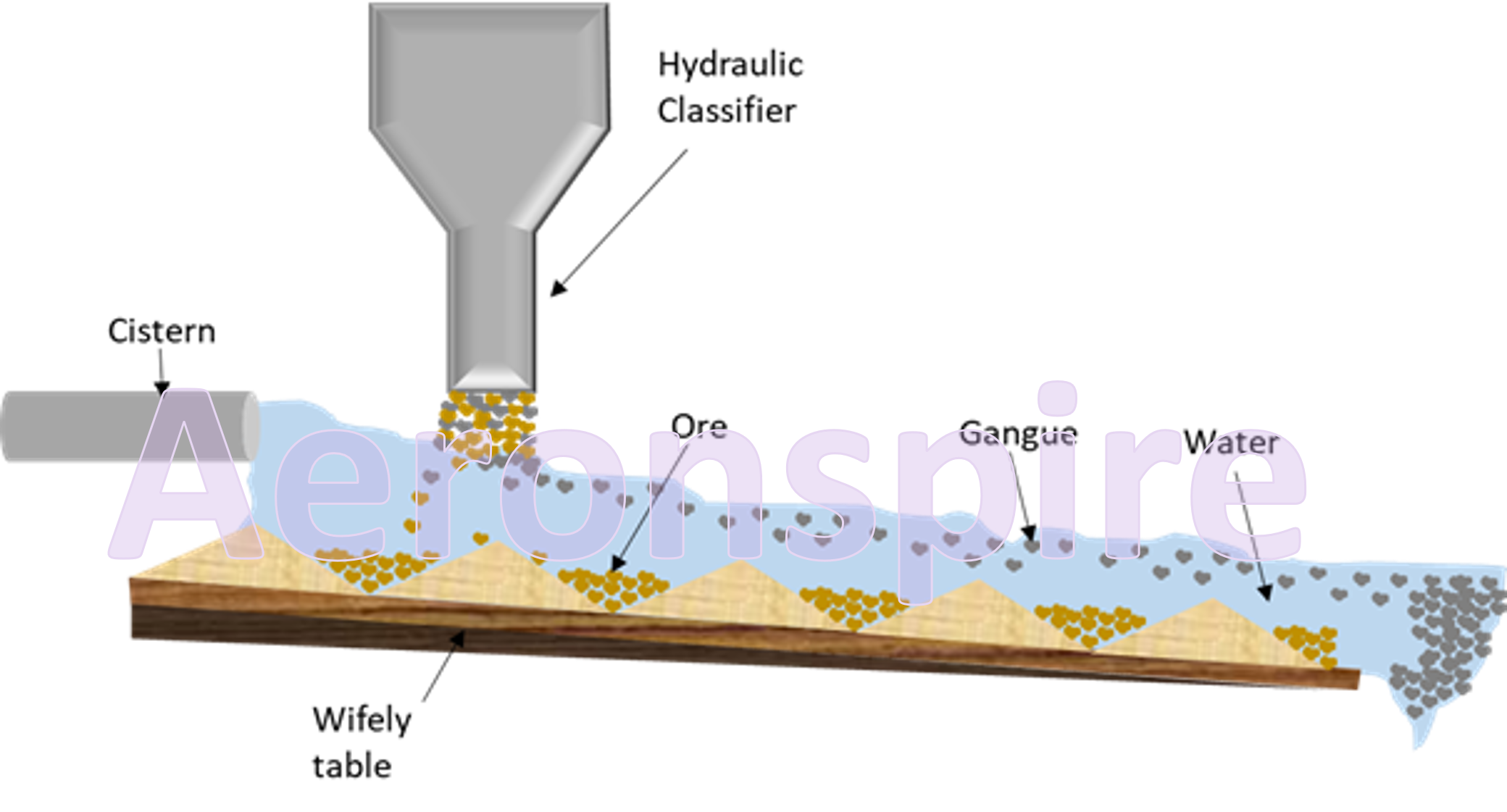
This
method is based on the differences in the specific gravities of metallic ores
and the gangue particles. Therefore, this method is known as gravity
separation. This method is frequently used when the ore particles are heavier
than the earthy or rocky gangue particles. In this method, the fine ore
collected after crushing is placed on a slopy surface (wifely table especially
designed for gravity separation method) and washed with a running stream of
water. The lighter impurities (gangue) are washed away, while the heavier ore
particles sink at the bottom of sloping platform. Generally, oxide ores like
haematite (Fe2O3) or tinstone (SnO2)
are concentrated by gravity separation method.
Electromagnetic separation
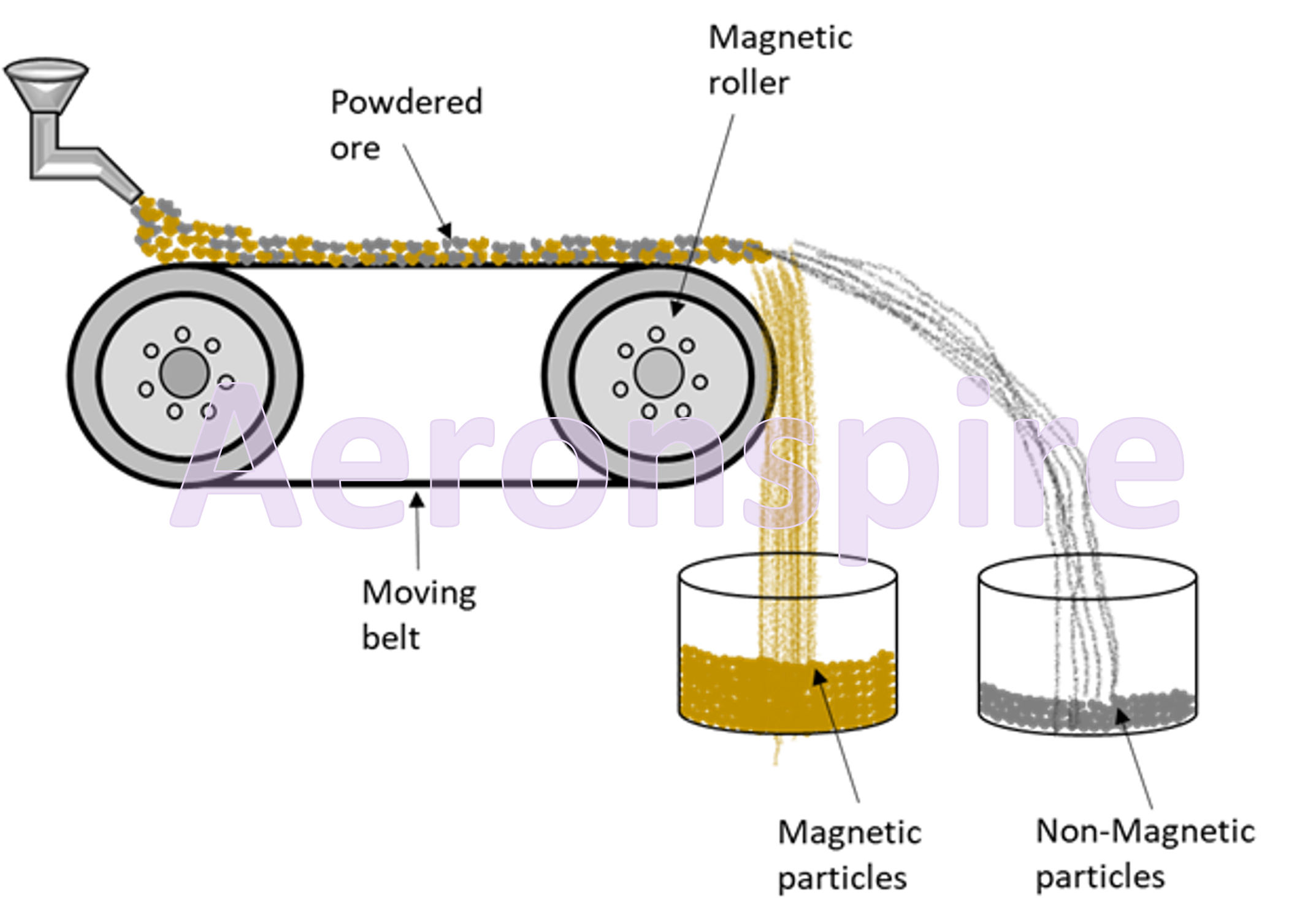
This
method is used for concentration of magnetic particles from non-magnetic
particles. This method is widely used for the separation of two minerals, when
one of them happens to be magnetic. The magnetic mineral can be separated from
the non-magnetic one by this method. For example, mixture of FeWO4
(magnetic) and cassiterite SnO2 (non-magnetic) are separated by this
method. The powdered ore is made to fall through a leather conveyor belt. The
conveyor belt is placed on a magnetic roll. The leather belt of conveyor moves
through electromagnetic roller. The magnetic impurities fall from the belt near
the roller due to its electromagnetic property while the non-magnetic
impurities fall far away from the roller.
Froth Floatation Process

This
method is extensively employed for the preliminary treatment of the minerals
especially sulphides. The process is based on the difference in wetting
characteristics of the gangue and the ore with water and oil. The ore is
preferentially wetted by the oil and the gangue particles by water. Particle
sized ore particles are mixed with water. The mixture obtained is called
slurry. A collector which acts as a surfactant (a substance which tends to
reduce the surface tension of a liquid in which it is dissolved) chemical
(usually mixture of water and pine oil) is added to the slurry, this is done to
enhance the hydrophobic nature of the mineral. The slurry has now been converted
into pulp. This pulp is added to the container filled with water and then air
jets are forced into it to create bubbles. The required mineral is repelled by
water and thus gets attached to the air bubbles. As these air bubbles rise up
to the surface with mineral particles sticking to them, these are called froth.
This Froth is separated and further taken for the next process of refining and
extraction.
Advantages
ü Almost
all types of minerals can be separated by this process.
ü Surface
properties can be controlled and altered by the flotation reagent.
Disadvantage
ü Result
can vary due to slime
ü Highly
expensive and complex
Chemical Process
Calcination
Metals
are usually obtained from oxide ores after going through the electrolysis or
reduction process. While oxide ores are easy to reduce, it is not the same with
carbonates and sulphides. These ores are turned to metals only after converting
sulphides and carbonates to an oxide ore. Calcination is the process of heating
ore strongly in the absence of air to a temperature insufficient to melt it.
The ore is heated below the melting
point either in limited supply or absence of air. Thus, calcination is mostly
used in the decomposition of limestone (calcium carbonate) to carbon dioxide
and lime (calcium oxide). Calcination is done in hearth of reverberatory
furnace (a furnace in which ores are heated by flames of a fuel) when the doors
are kept closed. The main purpose of calcination are:
ü
To remove carbonate
and hydroxide ore into oxide
Limestone: 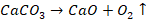
Malachite:
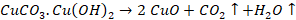
Haematite:
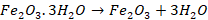
ü To
remove the moisture
ü To
remove the volatile impurities
ü To
make the mass porous, so that it can be easily reduced to the metallic state.
Roasting
Roasting
is a process of metallurgy where the ore is converted into its oxide by heating
it in the presence of excess air above its melting point. While calcination is
the process mostly used in the oxidation of carbonates, roasting is a method
that can be used for converting the sulphide ores. Roasting is done in the
hearth of a reverberatory furnace when the doors are kept open for the free
supply of air. This process is generally used in case of sulphide ores.
The
main purpose of roasting are:
ü To
convert sulphide into oxide and sulphate
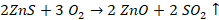
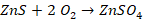
ü To
remove the moisture
ü To
remove volatile impurities like sulphur, arsenic, antimony and phosphorus in
the form of their oxides.
ü To
oxidise easily oxidisable substances
Difference between calcination and roasting
|
Calcination
|
Roasting
|
|
It
is the process of heating the ore to a high temperature in the absence of
air, or where air does not take part in the reaction.
|
The
process of heating the concentrated ore in the presence of air to a high
temperature so as not to melt is called roasting.
|
|
Usually
carbonate ores or ores containing water are calcined
|
Usually,
sulphide ores are roasted
|
|
Organic
matter, if present in the ore, gets expelled and the ore becomes porous.
|
The
impurities of P, As and S are removed as their oxides which being volatile,
escapes as gases.
|
|
It
is done in reverberatory furnace. The holes of furnace are kept closed.
|
It
is also done in reverberatory furnace but the holes of the furnace are kept
open to allow the entry of air into the furnace.
|
Reduction
The
oxide of the metal obtained as a result of Calcination or roasting is converted
into a free state by reduction. Reduction of metal oxide means removal of
oxygen to refine the metal to its free state. This is done with the help of
reducing agents such as Carbon (C), Carbon Monoxide (CO), Hydrogen (H2),
etc. Reduction can also be carried out by using electricity. Depending on the
type used, it can be classified in three processes i.e.
(a) Smelting
(b) Aluminothermic process
(c) Electrolysis
(a) Smelting
It is
the process of heating the ore or mineral above its melting point. In this
process, calcined ore is mixed with coke and flux like CaO and this mixture is
strongly heated above its melting point. The coke converts the ore into molten
metal while the gangue is removed in the form of slag. Smelting can be carried
out in blast furnace. It is also carried out in reverberatory furnace
sometimes. The flux added to remove the gangue from ore can be acidic or basic
in nature.
The hot
blast of dry air is blown into the furnace just above the hearth through a
number of pipes called twyers. In the well of the furnace there are two outlets
known as tap holes. Furnace charge is comprised of ore, coke, flux, etc. The
upper tap hole is used to remove slag and the lower one is used to remove the
molten metal. To remove the gangue in ore, certain oxides are added to the ore
while melting. These oxides are referred to as flux. If impurity is basic,
acidic flux is used and is impurities are acidic then flux used is basic. The
flux and gangue are converted to some fusible mass known as slag. This slag is
a fusible chemical compound formed by the combination of the added flux and
gangue present in ore.
For
example, in extraction of copper
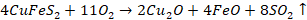
So FeO
is impurity and it is basic in nature. Now, to remove this impurity, we will
use acidic flux like SiO2. This flux and impurity will react to form
slag.
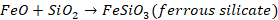
Similarly,
in extraction of iron, we have haematite ore
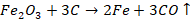
The
impurity is SiO2 which is acidic, so flux will be basic. Therefore,
limestone (CaCO3) will be used as flux. It decomposes at high
temperature to give lime. This lime reacts with silica to form slag.
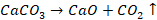
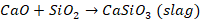
This
can be written as
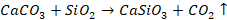
(b) Aluminothermic process
If the
oxides of a metal are very stable, aluminium is used as a reducing agent in
place of carbon at high temperature. Aluminothermic is a process of extracting
metals by reduction of a metal oxide to form metal using aluminium powder, the
aluminium acts as a reducing agent. It is an exothermic reaction which liberates
a large amount of heat. Practically, this method can be used to reduce all the
metallic oxides, with oxide of magnesium being the only exception.
Here
are few examples of Alumino-thermite process:
Reduction
of ferrous oxide to ferrous metal
Iron
(III) oxide is mixed with aluminium powder and is ignited with a burning
magnesium ribbon. Aluminium reduces iron oxide to produce molten iron metal
with the evolution of heat. The chemical reaction is gives as:
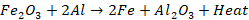
Cr2O3
is reduced to chromium metal
A
mixture of Chromic oxide and powdered aluminium in ratio of 3:1 and is ignited
by a piece of magnesium ribbon. A large amount of heat is liberated during this
process and chromic oxide is reduced to chromium. The chemical reaction is
gives as:
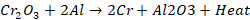
A
mixture of aluminium powder and metallic oxide in ratio 1:3 parts by weight are
known as thermite. Aluminium at high temperature has a great affinity for
oxygen. The reaction is exothermic; hence it liberates large amount of heat.
Metals obtained by this method are highly pure.
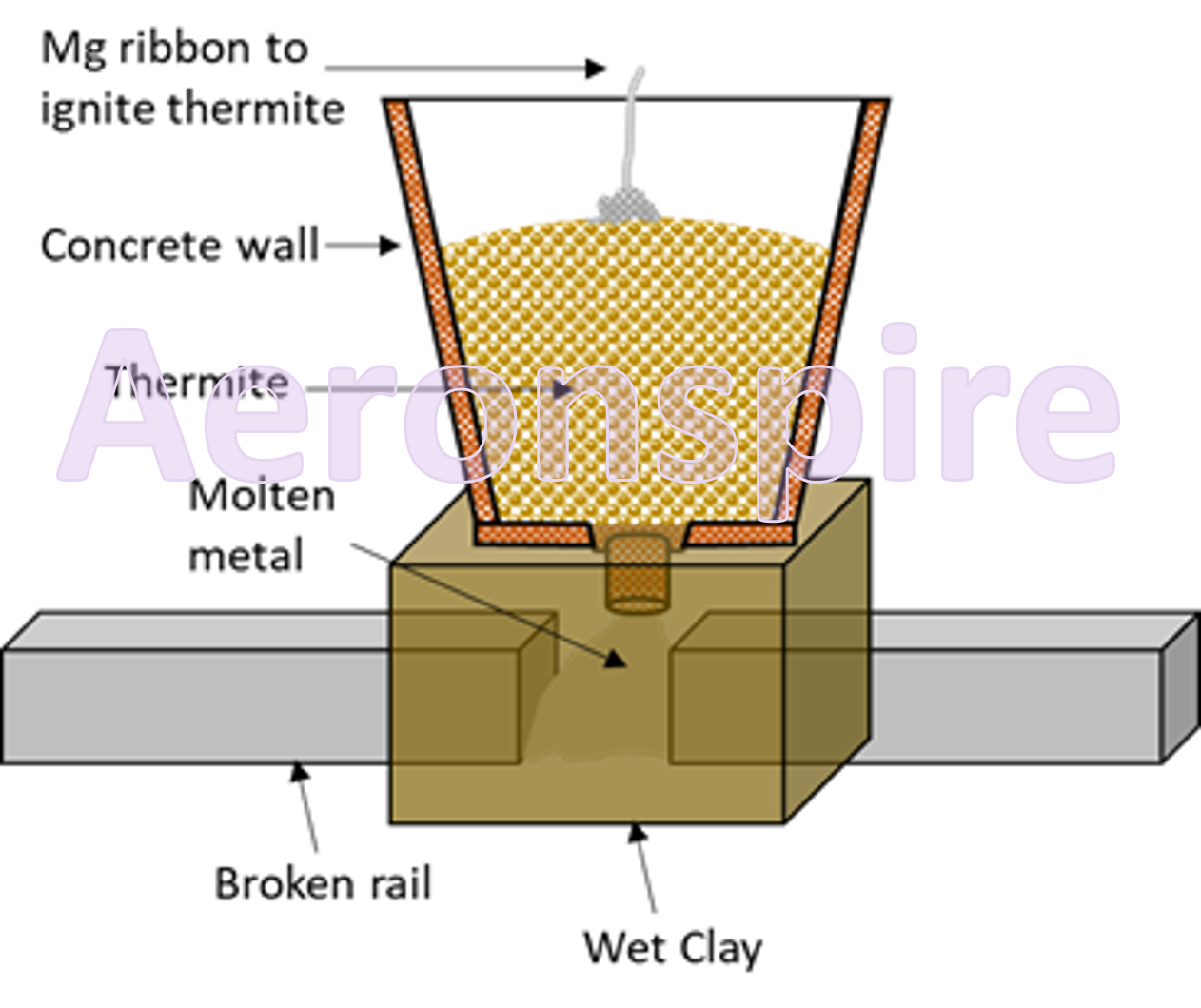
This
method is also used to weld the broken rails. The ends of the broken rails are
surrounded with a clay mould. A mixture of iron (III) and aluminium powder
(thermite) is ignited by magnesium ribbon in funnel above. The molten iron thus
obtained runs into the mould. This produces a perfect union upon cooling.
(c) Electrolysis
Electrolysis
is nothing but reduction of metal with the help of electricity. This method is
used to extract metal from oxides of very active metals. These metals form
strong bonds with oxygen, chlorine, etc. so that they are not reducible by
carbon.
These
metals cannot be obtained from their aqueous solution. If aqueous solution is
used, then H+ ions is discharged at cathode. This ion may will gain electron
and form H2 gas. The metals can only be obtained if we brought them
in their molten form. Suppose we want to extract Sodium from sodium chloride
(NaCl).

Two
electrodes are dipped in molten NaCl. These electrodes are then connected to
battery. The electrodes dipped are inert in nature. Now, the electrode
connected to negative terminal of battery will attract the Na+ ions.
Now Na+ will gain electrons and form solid sodium. Thus, we can say
that reduction takes place at cathode. Now, the electrode connected to positive
terminal of battery will attract the Cl– ions. These ions will lose
electrons at anode. Thus, we can say that, oxidation takes place at anode.
At
cathode, we have
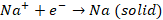
At
anode, we have
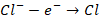
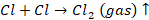
Refining
The
metal obtained from electrolysis is in pure form. So, it does not require
refining. But metals obtained from all the other processes apart from
electrolysis contains small amount of other elements. The process of
purification of metal to get extra pure metal is referred to as refining. There
are several methods to refine a metal. Some of them are as follows:
ü
Poling
ü
Liquation
ü
Distillation
ü
Electrolytic refining
Poling

Poling
is a method that includes a green log of wood to purify a metal. It is
typically used to purify metals like copper or tin that are in the impure form
of a copper oxide or tin oxide. A log of wood which is still green is used to
stir the liquid metal. The hydrocarbons in the green wood can reduce the metal.
The molten metal is placed in a concrete container. This hot crude molten metal
is stirred with green logs of wood. Also, during stirring, large quantities of
air is absorbed by the molten metal and such absorbed air oxidises the
oxidisable impurities. The oxidised impurities escape either as vapour or form
scum over molten metals. The scum thus formed is then removed by perforated
ladle.
Liquation
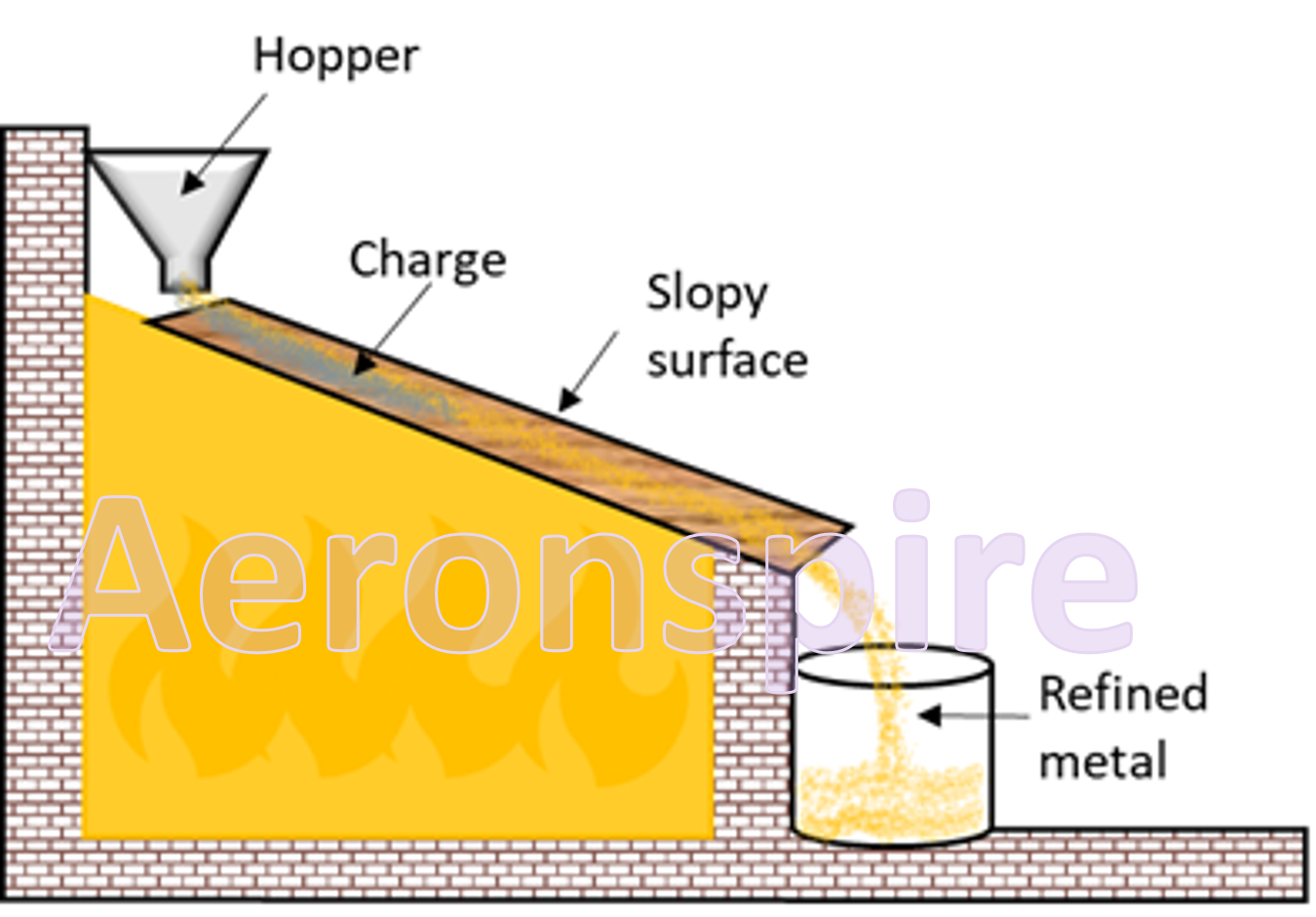
Liquation
is another method for refining of metal. This method is particularly suitable
for metal whose melting point is comparatively lower. The melting point of the
impurities is higher than the metal. The metals are converted into liquid
state by supplying heat at a temperature slightly above their melting point.
The crude metal is placed on a slopy surface. The metal with lower melting
point melts first and falls down the slope in a container. The impurities are
not melted and stays on slope. The impurities left behind are known as slope.
Distillation
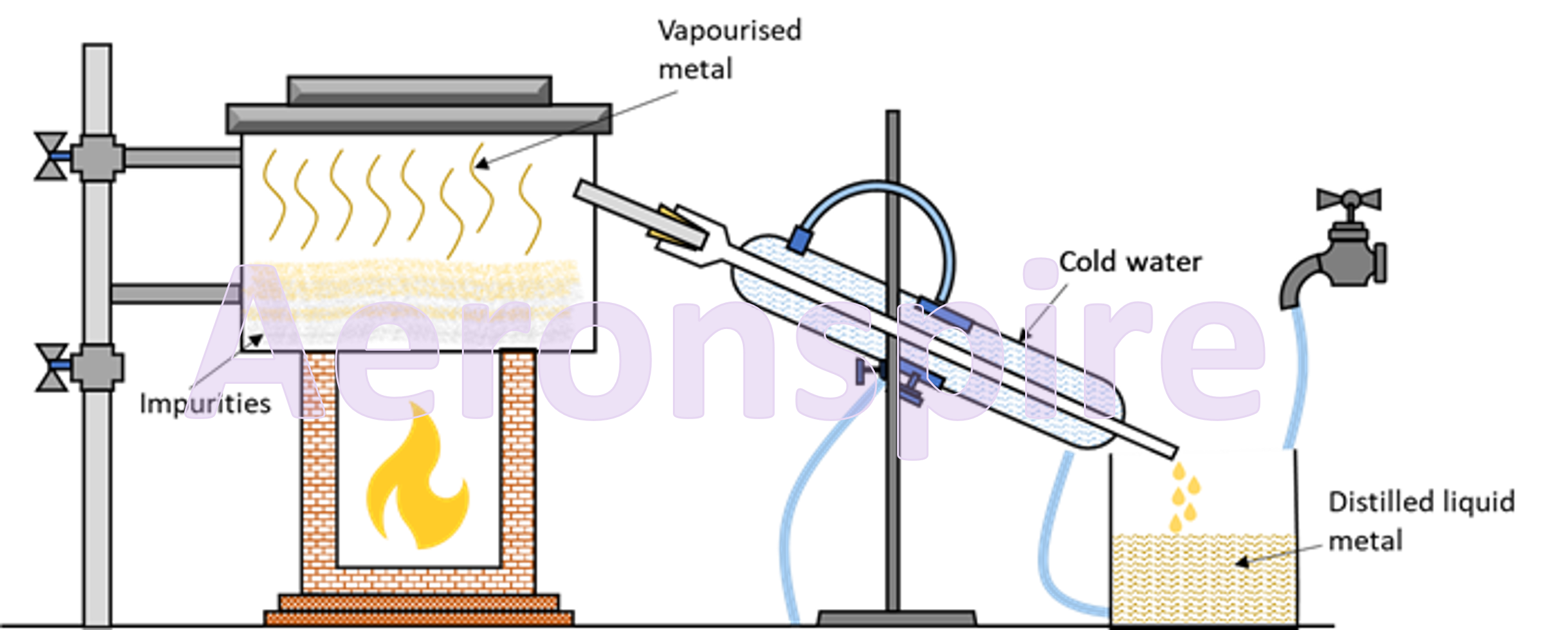
This
method is used for the purification of metals which possess a low boiling point
such as mercury and zinc. In this process, the impure metal is heated above its
boiling point so that it can form vapours. The impurities do not vaporise and
hence they are separated. The vapours of the pure metal are then condensed
leaving the impurities behind.
Electrolytic refining

Electrolytic
refining is a process of refining a metal (mainly copper) by the process of
electrolysis. As far as the mechanism of the process is concerned, during
electrolysis, a large chunk or slab of impure metal is used as the anode with a
thin strip of pure metal at the cathode. In this setup, an electrolyte (metal
salt aqueous solution) depending on the metal is often used.
The
clean or pure metal is formed at the cathode when the electrical current of a
sufficient voltage is applied by dissolving impure metal at the anode. When an
electric current with a definite voltage is passed through the bath, anode goes
on dissolving and only the pure metal is deposited on the cathode which grows
in size. Electrolytic refining is also sometimes referred to as
Electrorefining. Metals which are less electropositive than the one being refined
settle below the anode and is known as anode mud.
Metallurgy of Iron
Iron is
most widely used metal. It has occupied its space in our day to day lives like
buildings, ships, aeroplanes, etc. It is the 4th most abundant
element on earth after oxygen, silicon and aluminium. It is found in combined
state in nature such as oxide, sulphate, silicates, etc. Iron is also present
in our blood and it is very important for our body as well. It is found in
combined state in soil, rocks, etc.
The
important minerals of iron ore are as follows:
Oxides
Magnetite: Magnetite
is a very common iron oxide (Fe3O4 or FeO.FeO3)
mineral that is found in igneous, metamorphic, and sedimentary rocks. It is the
most commonly mined ore of iron. It is also the mineral with the highest iron
content (72.4%). It is magnetic and black in colour. It is also known as
Ferrosoferric oxide. This gives high quality of iron and mostly found in USA,
Sweden, Canada and India.
Haematite: Haematite
Fe2O3 is heavy and relatively hard oxide mineral. It is
red in colour. It is an important iron ore because of its high iron content
(70 percent) and its abundance. It is mostly found in USA, Brazil, China and
India.
Limonite: Limonite, one of the major
iron minerals, hydrated ferric oxide Fe2O3.nH2O.
It is brown in colour. The limonite ore was the lowest in grade of all the
oxides, with an iron content of 40%-60% percent. It is found in Belgium,
Germany, France and India.
Carbonate
Siderite
is a mineral composed of iron(II) carbonate (FeCO3). It is also
known as ferrous carbonate. It constitutes 40-45% of iron. It occurs in England
and West Bengal.
Sulphides
It is
found in form of iron pyrites FeS2, Copper pyrites CuFeS2,
Arsenical Pyrites FeAsS. The presence of sulphur in ore makes iron hard and
brittle and thus useless for many processes. But the sulphide ore is also rich
in copper, nickel, etc. So, this ore is not recommended for extraction of iron
but can be used to extract other metals.
Iron is
produced in three different commercial forms depending upon the number of
impurities present, especially carbon. They are:
Pig
iron: Iron obtained from reduction using carbon and
lime as reducing agent is basically pig iron or cast iron. In more general
term, an alloy containing more than 2% of carbon (other impurities are also
present) is called as pig iron. Pig iron is direct metallic product obtained
from blast furnace. It cannot be shaped into articles by forging or hammering.
It is therefore, melted and casted using moulds of desire shape. It is
therefore also referred to as cast iron.
Steel: Steel
is made from iron ore, a compound of iron, oxygen and other minerals that
occurs in nature. The raw materials for steelmaking are mined and then
transformed into steel using two different processes: the blast furnace/basic
oxygen furnace route, and the electric arc furnace route. Steel consists of
0.2% to 1.5% Carbon.
Wrought
Iron: Wrought iron is also known as malleable iron
and is very pure form of iron. It does not contain more than 0.5% carbon. It is
useful in application like chain, bolts, nails, etc. Wrought iron is composed
primarily of iron with 1 to 2% of added slag, the by-product of iron ore
smelting.
Extraction of iron
Extraction
of iron is mainly done in two major steps:
Step I: Preliminary
treatment
(a)
crushing and grinding
(b)
concentration
(c)
calcination or roasting
Step II: Smelting
(Reduction)
Preliminary treatment
The
first step in preliminary treatment is crushing and grinding.
(a) Crushing and grinding:
The ore
obtained in natural form is hard large bulky lumps. These lumps are first
broken into smaller pieces using a grinder. They
are broken to small pieces with the help of crushers or grinders. These pieces
are then reduced to fine powder with the help of a ball mill or stamp mill.
This process is called pulverisation.
(b) Concentration:
The
ores are usually found mixed up with large amounts of non-metallic impurities
such as, sand, mica, limestone, felspar, earthy and rocky impurities. These
unwanted impurities are called gangue or matrix and have to be removed before
extracting the metals. The process of removal of unwanted impurities (gangue)
from the ore is called ore concentration or ore dressing or ore benefaction.
The concentration of iron ore is done in two steps. Firstly, the ore is
concentrated using gravity separation method. In this method, the fine ore
collected after crushing is placed on a slopy surface (wifely table especially
designed for gravity separation method) and washed with a running stream of
water. The lighter impurities (gangue) are washed away, while the heavier ore
particles sink at the bottom of sloping platform. The mass then received free
from clay, sand and other earthy impurities are then dried and subjected to
magnetic separation. The conveyor belt is placed on a magnetic roll. The
leather belt of conveyor moves through electromagnetic roller. The magnetic
impurities fall from the belt near the roller due to its electromagnetic
property while the non-magnetic impurities fall far away from the roller. This
concentrates the ore upto 90% to 95%.
(c) Roasting:
The
oxidised metal is brought to its free method by roasting. The concentrated ore
is calcined or roasted in a reverberatory furnace at a low temperature, with a
little coke. Roastings brings following change to the ore:
Carbonate
ore is converted to oxide:
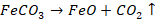
Ferrous
oxide is oxidized to ferric oxide and thus its conversion to ferrous silicate
(slag) is avoided
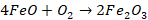
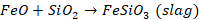
The
mass is dried up by removing moisture
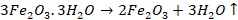
The
lighter non-metallic impurities such as sulphur, arsenic, phosphorus etc. are
all removed as volatile substances.
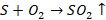
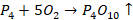
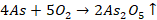
Ore
becomes porous and hence easy to reduce.
Reduction
The ore
is then reduced to highly concentrated iron.
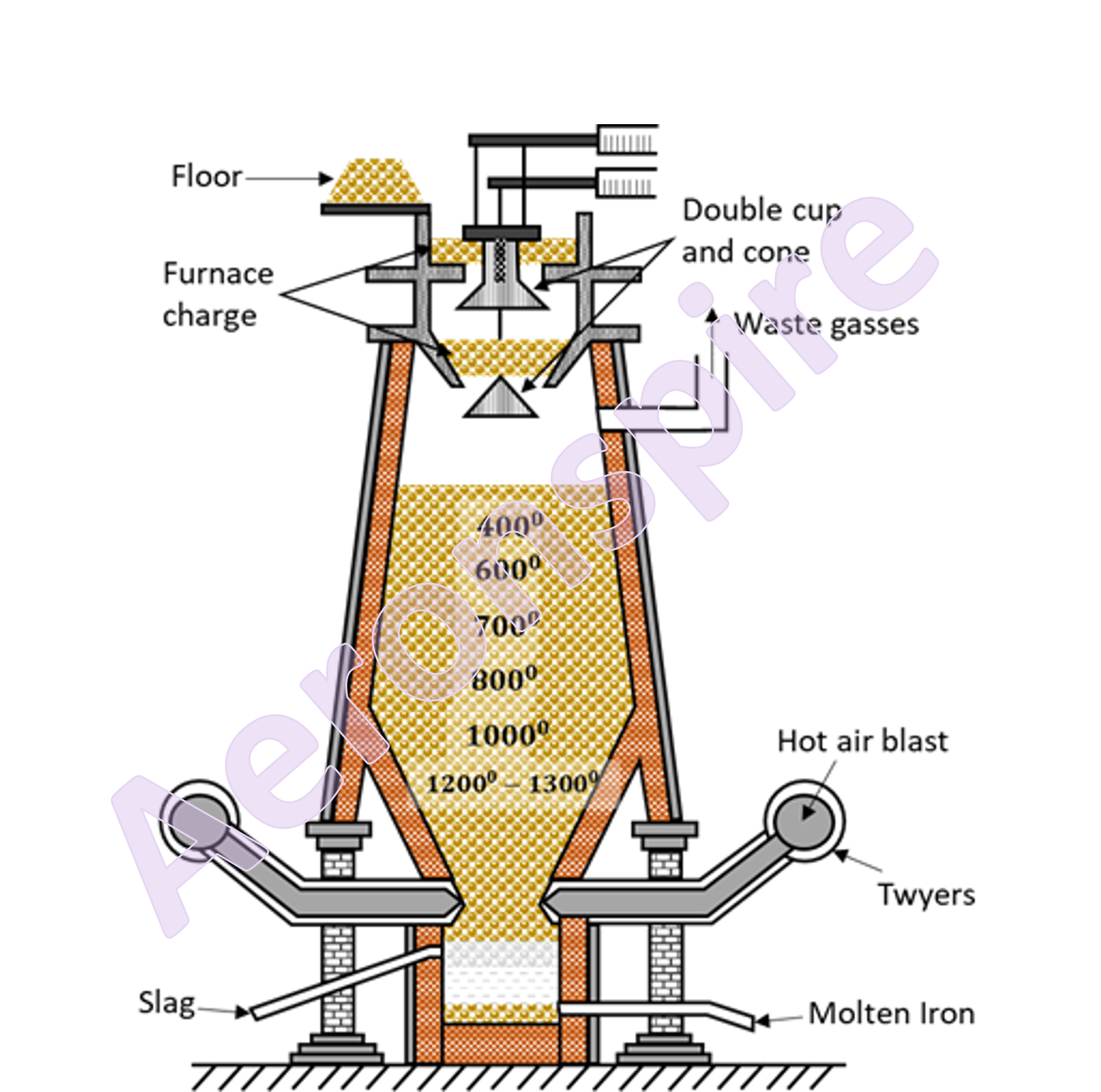
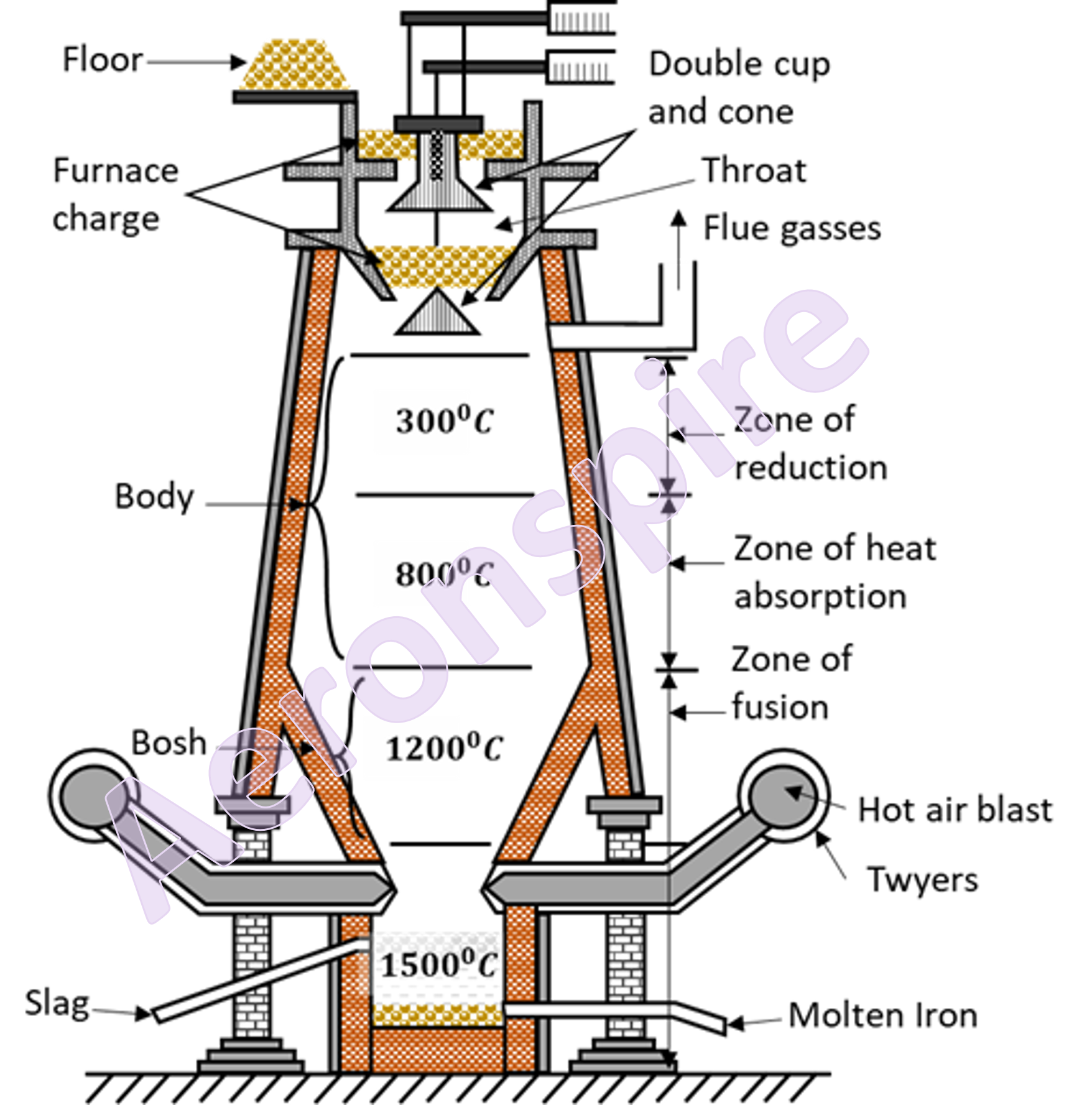
Smelting
The
process of reduction of iron ore to iron is done in blast process. The process
is known as smelting. The purpose of a Blast Furnace is to reduce the
concentrated ore chemically to its liquid metal state. A blast furnace is a
gigantic, steel stack lined with refractory brick where the concentrated iron
ore, coke, and limestone are dumped from the top, and a blast of hot air is
blown into the bottom. At the top, it is provided with double cup and cone
structure to permit charging without escape of waste gases. The upper part of
the furnace is called
the
throat, the middle part is known as body and the bottom part is referred to as
hearth. The region between body and hearth is bosh. The diameter of furnace
increases upto bosh. From the bosh downwards, the diameter decreases upto
twyers. A little above the base, the furnace is provided with series of water
jacketed pipes called twyers.
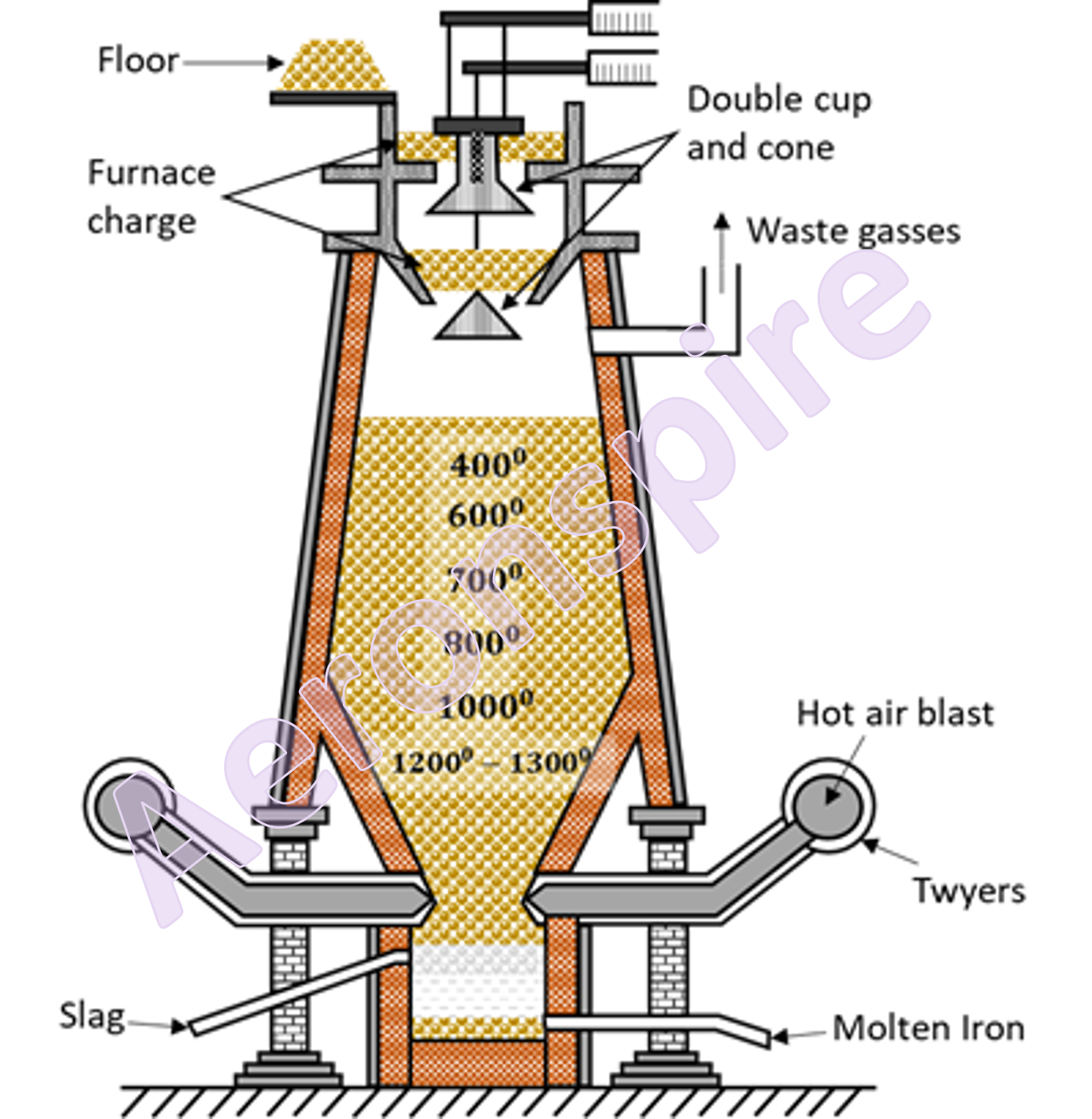
Working
The
charge is provided through double cup and cone mechanism. The charge
constitutes of calcined ore, de-sulphurised coke and limestone in ratio of
8:4:1. Various reactions takes place in blast furnace at different
temperatures. The reactions occur at different temperatures from 250o
C near throat to 1500o C near hearth. From throat to bosh, the
reaction takes place in three different zones as shown below:
(i)
Zone of reduction (300o C - 800o C)
(ii)
Zone of heat absorption (800o C - 1200o C)
(iii)
Zone of fusion (1200o C - 1500o C)
Zone of reduction (300o C - 800o C)
The
main reaction that occurs near the top of the furnace is reduction of the iron
oxide to metallic iron by carbon monoxide.
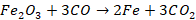
The
process of reduction continues as charge flows down the furnace. Series of
reaction takes place at different stages.
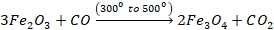
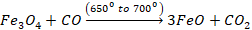
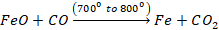
At the
same time, the limestone present in the charge is also decomposed to produce
lime
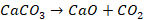
The
metal produced at first is spongy. Therefore, simultaneously with the process
of reduction, a part of metallic iron reacts with carbon monoxide to form
ferric oxide.
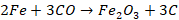
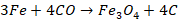
Zone of absorption (800o C - 1200o C)
In this
region, unreacted iron oxides get reduced to iron by red hot coke
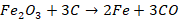
Carbon
monoxide gets disproportionate to CO2 and carbon powder
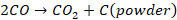
Lime
obtained at the end of first zone combine with impurities to produce slag
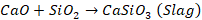
Other
impurities get reduced to the elementary substances and are mixed with finished
iron
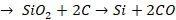
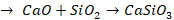
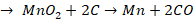
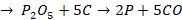
Zone of fusion (1200o C - 1500o C)
The
temperature of the bottom region is 1500o C. Iron melts in this
region. Molten iron gets collected through the bottom outlet. Slag also melts
at lower temperature and since it is lighter than iron, it floats over molten
iron and gets removed through another outlet. The molten iron is then sent into
sand moulds and cast into pigs or led to steel furnace and converted into
different type of steels. The output from blast furnace is cast iron or pig
iron whose composition is given below:
|
Iron
|
92%
to 95%
|
Phosphorus
|
0.5%
to 1%
|
|
Carbon
|
2.5%
to 4.5%
|
Manganese
|
0.2%
to 1%
|
|
Silicon
|
0.7%
to 3%
|
Sulphur
|
0.1%
to 0.3%
|
This
pig iron can then be converted to steel or wrought iron as per requirement.
When the molten iron is cooled suddenly, it is known as white cast iron
but when it is cooled slowly, it is known as grey cast iron.
Metallurgy of Copper
Copper
occurs in native as well as in combined state. Most common ores of copper are
in form of sulphides, carbonate and oxides.
Sulphide Ores: Copper
Pyrite (CuFeS2) or copper glance Cu2S
Oxide ores: Cuprite
or ruby copper (Cu2O)
Carbonate ores: Malachite
(CuCO3, Cu(OH)2) or azurite 2CuCO3
The
commonest ore used in the extraction of copper is Chalcopyrite (CuFeS2)
also known as Copper Pyrites and other such sulphides.
The
steps involved in extraction of copper
Step1: Crushing
Step2: Concentration
Step3: Reduction
Step4: Bessemerisation
Step5: Refining
Crushing
The
copper pyrite ore is crushed in a big jaw crusher. The output from crusher is
not fine. Therefore, it is passed through a stamping mill. The stamping mill
convert smaller lumps of ore to finely powdered ore.
Concentration
The
process of removal of unwanted impurities (gangue) from the ore is called ore
concentration or ore dressing or ore benefaction. The concentration can be done
either by a physical method or chemical method.
Concentration by physical method (Froth floatation method)
In
physical method no chemical reaction takes place during concentration. The ore
is concentrated using physical property. The finely powdered ore can be
concentrated by froth floatation method. This method is extensively employed
for the preliminary treatment of the minerals especially sulphides. Particle
sized ore particles are mixed with water. The mixture obtained is called
slurry. This is added to the container filled with water and then air jets are
forced into it to create bubbles. The required mineral is repelled by water and
thus gets attached to the air bubbles. As these air bubbles rise up to the
surface with mineral particles sticking to them, these are called froth.
Concentration by chemical method (Roasting)
For
concentration of ore by chemical method, it requires some chemical changes to
ore. This can be done using roasting method. During roasting following chemical
reactions occur
Copper
pyrites decompose to form cuprous and ferrous sulphides
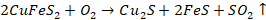
A part
of these sulphides gets oxidised to corresponding oxides
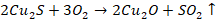

Moisture
is eliminated and impurities like sulphur are removed in their volatile oxides
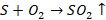
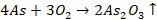
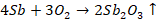
Reduction
Reduction
of copper ore is done by smelting. The roasted ore is mixed with coke and sand
and is heated in blast furnace in the presence of excess air. Copper Smelting
means that the concentrated ore is heated strongly with silicon dioxide
(silica), calcium carbonate (CaCO3) and air in a furnace. The major
steps in the extraction of copper are
ü Copper
in Chalcopyrite is reduced to copper sulphide.
ü Calcium
carbonate is added as a flux to create the slag.
ü Iron
in Chalcopyrite is removed as iron silicate slag.
ü Most
of the sulphur in Chalcopyrite turns into Sulphur dioxide (SO2).
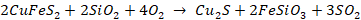
The
copper extracted from this process is mixed with the slag and is called Matte
Copper due to its texture and appearance. Matte is a mixture of molten Cu2S
+ FeS. This molten matte so produced is then converted to blister copper by Bessemerisation.
Bessemerisation
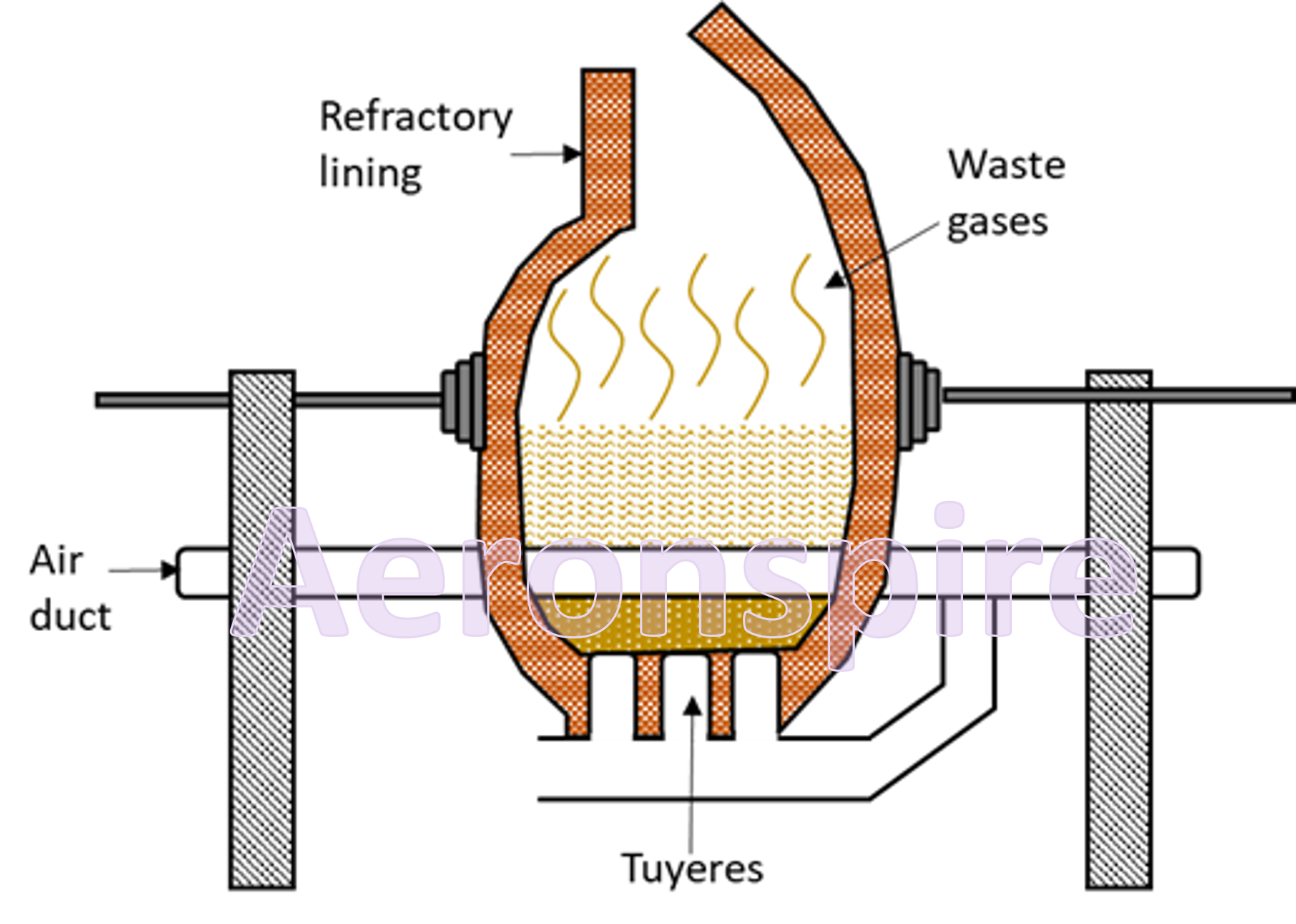
The
molten metal is then transferred to a Bessemer converter. Bessemer converter is
a furnace made up of steel plates and lined with basic lining of lime or
magnesia. It is mounted on pivot and can be tilted in any position. The furnace
is provided with pipes known as twyers through which sand and hot air is blown
into it. The tuyeres are fitted sufficiently high above the bottom so that the
molten metal drops below the level of twyers and escapes the oxidising action
of the air. FeS is converted to slag. Slag is poured off by tilting converter. Cu2O
is reduced to metallic copper.

After
the reduction is complete, the molten copper is poured into sand moulds. As the
metal solidifies, SO2 escapes and leaves blisters on the surface.
The solid metal thus obtained is called as blister copper which contains 96% to
98% copper. The remaining 2% is iron with small amounts of An, Ni which were
present originally in the ore. This copper can be used in construction of
pipes, boilers, etc. But to conduct electricity copper must be extremely pure.
There blister copper goes to refining stage.
Refining
Refining
of copper can be done in two stages as discussed below to obtain extremely pure
copper. However, either of the stages can be used for refining of copper.
Poling
In this
process, blister copper is melted in a reverberatory furnace in the presence of
air. The molten mass is then stirred with a green log of wood. The green log of
wood produces hydrocarbon gases which reduces cuprous oxide to metallic copper.
This refining reduces copper with 99.2% to 99.6% concentration
Electrolytic refining
The
copper obtained from poling can be concentrated up to 99.9% by electrolytic
refining. However, blister copper can be directly refined using this method.
This is a standard electrolysis setup, where the impure copper (the sample to
be refined) is placed as anode and a thin strip of pure copper is placed as a
cathode.

Impure
or blister copper is about 99% pure when extracted from the ore. Copper metals
can be refined up to 99.99% by electrolytic refining. The anode (positive
electrode) is made from impure copper, and the cathode (negative electrode)
from pure copper. Copper sulphate acidified with sulphuric acid is used as an
electrolyte in this process. By passing electric current, the impure metal
dissolves from the anode into the electrolytic solution. In this phase, copper
sulphate dissociates into Cu++ and SO42- ions.
The positive copper ions, also known as the cations, move towards the cathode,
which is made up of pure copper metal. The metal cations absorb electrons from
the cathode and get deposited on the cathode as Cu atoms. Thus, pure copper is
produced at the cathode.
The
following reactions take place when an electric current is passed through the
electrolytic solution:
At
cathode:
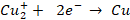
At
anode:
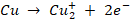
The
soluble impurities dissolve in the electrolytic solution, while insoluble impurities
can be found at the bottom of the anode. This insoluble impurity is known as
anode mud. In this method, the cathode is coated with graphite as it makes the
separation of pure copper easier.
Properties of copper
Engineering
properties of copper
Copper
is a heavy reddish-brown metal. It gets polished easily and hence used for
making utensils.
ü It
has high melting point and boiling point. Also, it is a very good conductor of
electricity thus making it useful for electrical connections
ü It
is malleable and ductile and can be drawn into wires of small diameter
ü Copper
is next to silver in terms of conductivity to heat and electricity
ü It
casts very well and forms alloys
Chemical
properties of copper
Action of air
Copper
is not attacked by dry air at ordinary temperatures. However, if it is heated
till it becomes red in the presence of air, it forms cupric oxide and on
further heating it forms cuprous oxide.
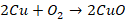
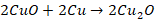
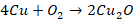
Action of water
Water
at ordinary temperature has no action on copper. However, red hot copper reacts
with steam and form oxide
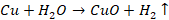
Action of acid
Action of HCl (hydrochloric acid)
Cold
and dilute HCl has no action on copper. Copper reacts with warm dilute HCl in
presence of oxygen to form cupric chloride
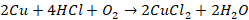
Also,
copper powder dissolves slowly in hot and concentrated HCl with the evolution
of hydrogen
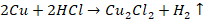
Action of H2SO4 (sulphuric acid)
Cold
and dilute H2SO4 has no action on copper. It dissolves
slowly in hot and diluted H2SO4 in the presence of
oxygen.
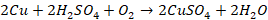
On heating
with concentrated H2SO4, it produces SO2
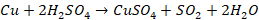
Action of HNO3 (nitric acid)
Dilute HNO3
dissolves the metal with the evolution of nitric acid
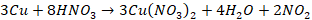
Concentrated
HNO3 dissolves the metal and gives nitrogen dioxide
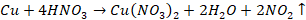
Action of CH3COOH acetic acid
In
presence of air, copper reacts with acetic acid to form cupric acetate
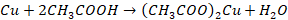
Metallurgy of aluminium
Aluminium
is a soft, silvery-white, corrosion-resistant metal. It is the most abundant
metal in the earth’s crust as it makes up 8% of the crust and it is the third
most abundant element after oxygen and silicon. It occurs widely as constituent
of rocks and soils. Its main ores are:
Oxides: Bauxite (Al2O3.2H2O),
Corundum (Al2O3)
Fluoride: Cryolite
(Na3AlF6)
Silicates: Feldspar (KAlSi3O8),
Mica (KAlSi2O10(OH)2)
Basic sulphates:
Alunite (K2SO4.Al2(SO4)3.4Al(OH)3)
Bauxite
ore is most widely found and hence it is commonly used to extract Aluminium.
Extraction of aluminium
Extraction
of aluminium involves following three steps:
Step1: Purification of Bauxite
Step2: Electrolytic
reduction of alumina
Step3: Refining
of alumina
Purification of bauxite:
Bauxite
ore generally contains ferric oxide and silica as impurities. Dressing of
Bauxite ore is done by crushing and pulverising. Purification method varies
according to the type of impurities present.
ü Baeyer’s
process for the removal of impurities of iron (red bauxite)
ü Serpeck’s
process for the removal of impurities of silica (white bauxite)
ü Hall’s
process for the removal of both iron and silica impurities
Baeyer’s Process
The
powdered bauxite ore is roasted to convert ferrous oxide (FeO) to ferric oxide
(Fe2O3). This roasted ore is then heated with
concentrated NaOH under pressure for few hours in autoclave. This process is
referred to as leaching. Aluminium oxide dissolves forming sodium
meta-aluminate, while ferric oxide remains undissolved.
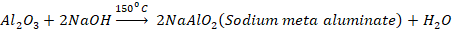
Undissolved
Fe2O3 is removed by filtration. The sodium meta-aluminate
is diluted with water to form a precipitate of aluminium hydroxide Al(OH)3.
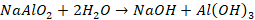
The
precipitate of Al(OH)3 is then filtered out, dried and heated at 1500℃
to get pure alumina
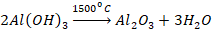
Serpeck’s process
Serpeck
process is a method of purification of bauxite ore containing silica (SiO2)
as the main impurity. When Silica is the main impurity, it is called
white bauxite. The powdered bauxite is mixed with carbon and heated up to 1800℃
and a current of nitrogen is passed, aluminium nitride is obtained.
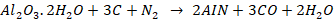
Aluminium
nitride thus obtained is hydrolysed with water to get a precipitate of Al(OH)3.
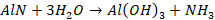
The
precipitate of Al(OH)3 is filtered, washed and dried.
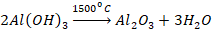
The
silica present as an impurity in bauxite is reduced to silicon which is volatile
at high temperature. So, it is removed easily.
Hall’s Process
This
method is used for the ore having both Fe2O3 and SiO3
as impurities. The powdered ore is bright red heated with sodium carbonate.
Aluminium oxide dissolves to form sodium meta-aluminate while insoluble Fe2O3
and SiO2 are left residue.

The
fused mass is extracted with water and the insoluble Fe2O3
and SiO2 are removed by filtration. The filtrate is then heated to 50o
C to 60o C and a current of CO2 is passed through it and Al(OH)3
is precipitated out.
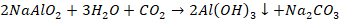
Al(OH)3
is filtered off, dried and ignited to get pure alumina.
Reduction
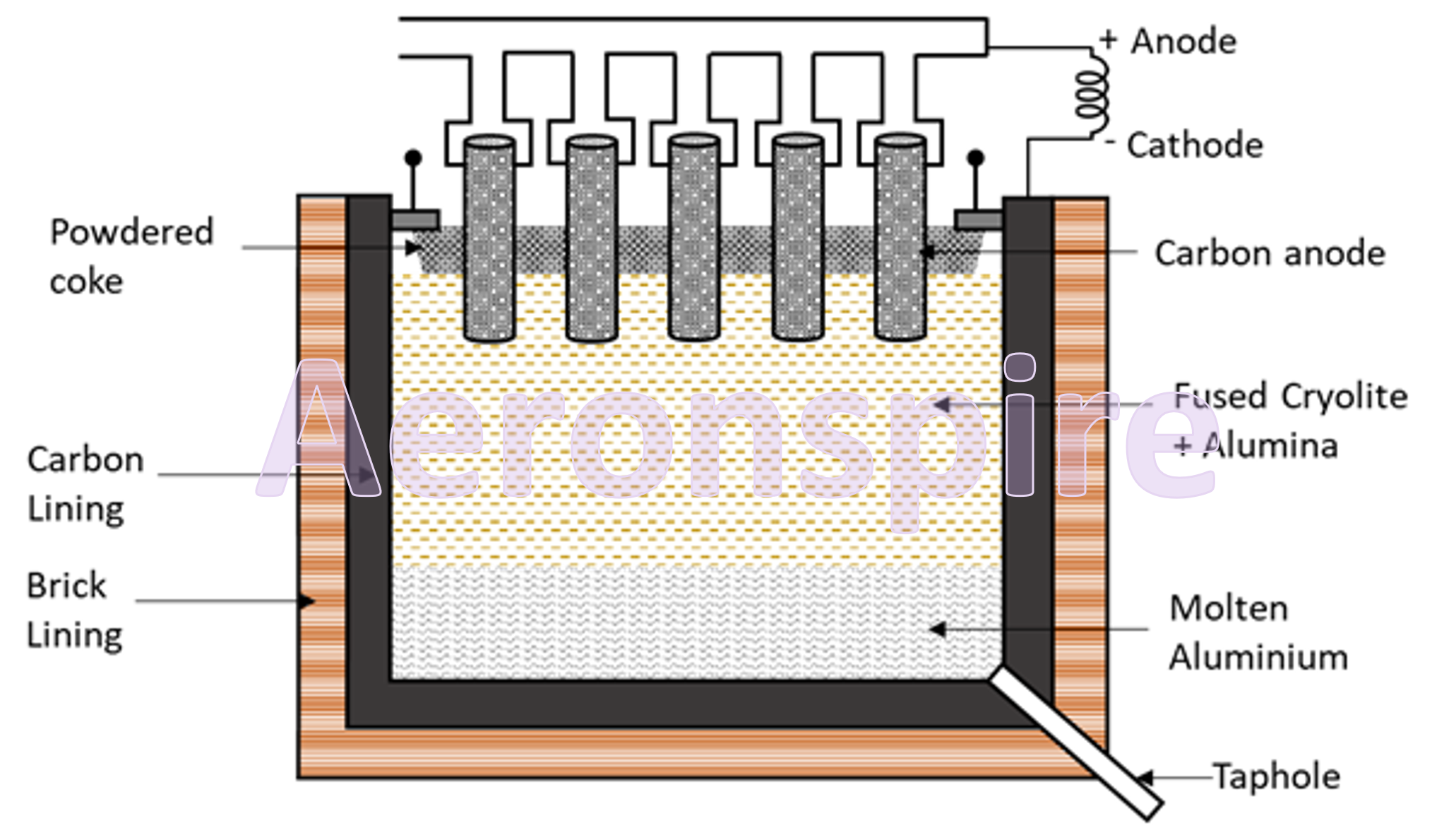
Alumina
cannot be reduced to metallic aluminium by carbon, because aluminium has great
affinity for oxygen and thus the reduction of alumina by carbon under ordinary
condition is not possible. Thus, alumina is reduced to aluminium using
electrolytic reduction. However, alumina is a bad conductor of electricity and
has very high melting point. If electrolysis is carried out at 2000o
C, aluminium obtained vaporises. Hall and Heroult overcome these difficulties
by carrying the electrolysis of alumina in the presence of cryolite (Na3AlF6)
which acts as a proper electrolyte. In Hall-Heroults process, pure Al2O3
is mixed with CaF2 or Na3AlF6. This results in
lowering the melting point of the mixture and increases its ability to conduct
electricity. A steel vessel with a lining of carbon and graphite rods is used.
The
carbon lining acts as a cathode and graphite act as an anode. When electricity
is passed through the electrolytic cell which consists of carbon electrodes
oxygen is formed at the anode. This oxygen formed reacts with the carbon of the
anode to form carbon monoxide and carbon dioxide. In this
method of production of aluminium, for every 1 kg of Al produced, approximately
0.5 Kg of carbon anode is burnt. This electrolytic bath is covered with
powdered coke to avoid the oxidation of metal and to avoid the loss of heat.
Heat produced by the current keeps the mass in fusible state. The temperature
of bath is maintained at 900o C – 1000o C. On passing
current, alumina decomposes to aluminium and oxygen

During
the process of electrolysis, Aluminium ions that are positively charged gain
electrons from the cathode and form molten aluminium. Oxide ions lose electrons
at the anode and form molecules of oxygen.
The
molten aluminium sinks to the bottom (cathode) from where it is tapped off from
time to time. Oxygen is liberated at anode, which then combines with carbon
anodes and form CO and CO2. These gases escape through outlet.
At the
cathode:
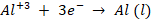
At the
anode:
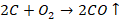
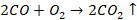
The
concentration of alumina falls in the cell below a certain limit, the
resistance of the cell increases and therefore, more current flows through lamp
connected in parallel with cell and it glows. This indicates that alumina is
exhausted and more alumina needs to be added to continue the process.
Refining of aluminium
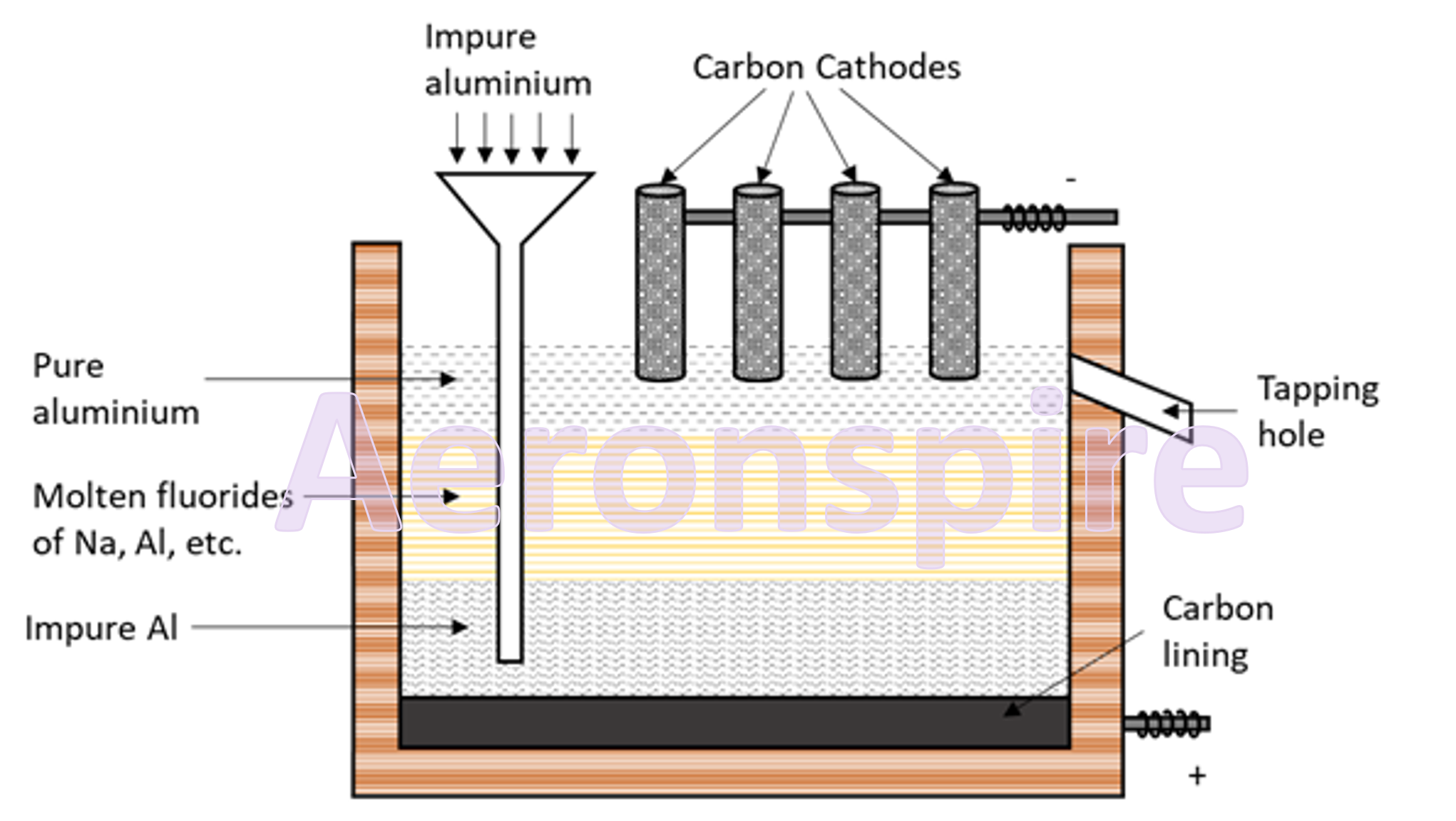
Aluminium
is further purified by Hoope’s process. The electrolytic cell consists of an
iron box lined inside with carbon. The cell consists of three layers which
differ in specific gravities.
a)
The upper layer is of pure aluminium which acts as cathode.
b)
The middle layer consists of a mixture of the fluorides of Al, Ba and Na.
c)
The lowest layer consists of impure aluminium which acts as anode.
When
electrolysis occurs, current passes through the middle layer and the Al+3
ions migrate from the middle layer towards the cathode where they get reduced
to form Al. The lowermost layer or the anode produces an equal number of Al+3
ions, which move to the middle layer and then to the upper layer where they
form Al.
In this
way all the pure aluminium gets deposited at the uppermost layer and the
impurities are left behind. The aluminium obtained at the end was 99.99% pure.
Properties of aluminium
Physical properties
ü Aluminium
is silvery and lustrous white metal which takes very high polish.
ü Aluminium
is a good conductor of heat and have high melting and boiling point and thus is
used to make utensils
ü Aluminium
is a good conductor of electricity and thus used to make wire
ü It
is malleable and ductile and can be rolled into sheets, foils and wires.
Chemical Properties
Action
of air
Aluminium
is not affected by dry air and form a thin layer of oxide in moist air. This
prevents aluminium from further corrosion. On heating in air or oxygen, it
burns readily which produces light with the evolution of a huge quantity of
heat.
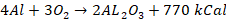
Action
of water
Pure
aluminium is not affected by pure water. However, impure aluminium is readily
corroded by water containing salts. It decomposes boiling water with the
evolution of hydrogen.
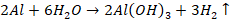
Amalgamated
aluminium Al - Hg reacts most instantly with cold water liberating hydrogen.
Hence it is used as reducing agent.
Action
of acid
Aluminium
dissolves readily in HCl to form aluminium chloride and liberates hydrogen.
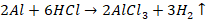
Diluted
H2SO4 reacts slowly with aluminium and liberates hydrogen
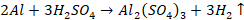
Hot and
concentrated H2SO4 liberates SO2 gas with
aluminium

Nitric
acid has no action on pure aluminium
Action
of base
Aluminium
readily dissolves in strong bases like NaOH, KOH forming metal aluminates and
liberates H2 gas.
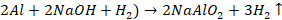
It
dissolves in hot concentrated solution of Na2CO3 forming
sodium meta-aluminate
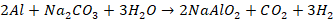
Alloys
The
meaning of the term ‘alloy’ is a substance formed from the combination of two
or more metals. Alloys can also be formed from combinations of metals and other
elements. Pure metals have properties like metallic lustre, good electrical
conductivity, high malleability, and ductility. These metals are very soft,
highly chemically reactive. This makes metal useless for engineering purposes.
To make metal useful, desired properties are incorporated by mixing it with
other compounds. To impart certain properties to metals, or in order to
strengthen some of their existing properties, certain other metals/elements can
be added to the metals in specific ratios to form alloys. For example, pure
aluminium is a relatively soft metal. Pure copper is also quite soft. However,
when aluminium is alloyed with copper, the strength of the resulting alloy is
far greater than that of its parent metals.
Properties of alloy
An
alloy’s properties are distinct from those of the individual metals from which
it is produced. Some properties of alloys are given below.
ü An
alloy is a homogeneous mixture of two or more elements one of which must be a metal.
ü Alloys
are harder than their constituent metals.
ü Alloys
are more resistant to corrosion than pure metals.
ü Alloys
are more durable than the metals they are made from.
ü The
electrical conductivity of alloys is lower than that of pure metals.
ü Alloys
have a lower melting point than the metals from which they are made.
ü Alloys
have greater ductility than their constituent metals.
Preparation of alloys
An
alloy can be prepared by any of the following method
(a) Fusion
(b) Electro deposition
(c) Compression
(d) Reduction
(a) Fusion
This
method uses alloying elements in a fixed proportion and fuses them together in
a refractory melting pot or in a brick-lined crucible. The component metal with
a higher melting point is melted first and then the other component with a
lower melting point is added to the melt. Both metal components are mixed well
and allowed to melt further. The molten mass is covered by powdered Carbon to
avoid oxidation of the molten alloy components because they are very reactive
to the surrounding atmospheric oxygen. The resulting molten mass is allowed to
cool at room temperature. In the production of brass, an alloy of copper and
zinc is melted first and then required quantity of zinc is added to it which
melts immediately. The molten mass is stirred, covered with charcoal, and
allowed to cool to avoid oxidation of copper and zinc.
(b) Electro deposition
This
method involves simultaneous deposition of different component metals from the
electrolytic solution containing their salts solution mixture by passing direct
electricity. Brass can also be obtained by electro deposition method. The
electrolysis of mixed solution of copper and zinc cyanides dissolved in
potassium cyanide is carried out to obtain brass.
(c) Compression
Alloy
can also be made by method of compression. In this method, two or more metal
powders are mixed and compressed under a high pressure in a mould. The moulded
article is then heated to a temperature just below the melting point of an
alloy. Due to this, tiny particles of metal are firmly welded to one alloy. An
alloy of lead and tin known as solder alloy is made by this method. Wood’s
metal is an alloy of lead, tin, bismuth, and cadmium is also made by this
method.
(d) Reduction
The
alloy is obtained by the reduction of a suitable compound, generally oxide of
one component metal in the presence of the other component metal. The component
metal oxide is reduced to metal in the presence of other metal. Aluminium
bronze is alloy which is prepared by reducing aluminium oxide in the presence
of copper in an electric furnace.
Need of alloys
To improve the hardness of metal: Pure metals are generally soft. But when
the metal is alloyed with another metal or non-metal, its hardness is
increased. Pure gold and silver are soft and hence cannot be used. But to make
it useful, it is hardened by addition of small amount of copper. Similarly,
pure iron is very soft and cannot be used for any engineering purposes. The
iron is hardened by addition of small amount of carbon. The resultant iron is
nothing but steel.
To lower the melting point: When an alloying element is added to base
metal, it acts as an impurity. The impurity lowers the melting point of base
metal. Wood’s metal is an alloy of bismuth, lead, tin, and cadmium has melting
point of 70o C.
To increase the tensile strength:
While a metal is alloyed, it increases its tensile strength. For example, when
iron is alloyed with 1% of carbon, it increases its tensile strength by 10
times.
To increase the corrosion resistance: The metals in pure form are highly
reactive. They get easily corroded with atmospheric air and moisture. But if
metal is alloyed, it resists corrosion. For example, iron gets easily corroded
in air and moisture, but its alloy steel resists corrosion.
To get good casting: Pure
metals contract on solidification so it becomes difficult to cast fine
impression. Hence, in order to get good castings, metals must be alloyed. Alloy
expands on solidification.
To reduce malleability and ductility:
Pure metals are highly malleable and ductile. Pure metals change their shape
even when very small force is applied. Its malleability and ductility can be
reduced by making it alloy.
To modify chemical activity: Pure metals are highly reactive and can
easily react with other elements. But when it is alloyed with other element it
becomes less reactive. For example, sodium is highly reactive but when alloyed
with mercury (sodium-amalgam (Na-Hg)) it becomes less reactive.
Classification of alloys
Alloys
are generally classified in two classes
Ferrous
alloys
Non-
ferrous alloys
Ferrous alloys: Ferrous
alloys are those alloys which contains iron as its main component. The term
‘ferrous’ has been derived from the Latin term ‘ferrum’ meaning iron. The most
common ferrous alloys are steel.
There
are Different Forms of Ferrous Metals Available in the Market. Some of the
Major Types and Their Characteristics are Stated below:
Stainless
Steel – Resistance to Corrosion
Cast
Iron - Hard, brittle, strong, self-lubricating,
economical
Mild
Steel – Ductile, tough, high tensile strength. Due to
low carbon content, it cannot be toughened and tempered. It should only be case
hardened.
High
Carbon Steel - The hardest of the carbon steels. Tough
and malleable but less ductile.
Methods of steel making
Steel
is manufactured by blowing hot air or oxygen through molten cast iron whereby
impurities are oxidized and removed as volatile gases. Small amount of carbon
is added to the pure molten iron to make steel of required strength and
malleability. Steel can be made by any of the following methods: Electric
process, Bessemer process, Open hearth process and duplex process. These are
old methods of making steel. New methods include Kaldo process and
Linz-Donawitz process.
Bessemer Process
The
Bessemer process was the first inexpensive industrial process for the mass
production of steel from molten pig iron before the development of the open
hearth furnace. The key principle is removal of impurities from the iron by
oxidation with air being blown through the molten iron. In this method, a
furnace called Bessemer converter is used. It is a pear or egg shaped vessel
constructed by using steel plates. It is supported on side arms called trunnion
to orient it in different angles. Twyers are provided at the bottom and hence
it is also known as bottom-blown converter. From inside it is lined with
refractory lime bricks or silica bricks. When acidic impurities are to be
removed, basic lining such as dolomite (CaCO3.MgCO3) is
used and the process is referred to as basic Bessemer process. When basic
impurities need to be removed, acidic lining of silica is used and the process
is referred to as acid Bessemer.
Acid Bessemer process
In this
process the converter is lined with silicious refractory material. The
converter starts in horizontal position and charged with about 60 tonnes of
molten cast iron at 1200o C. Then it is turned to vertical position and
a hot blast of air blown from the twyers. Impurities get oxidized and
temperature rises to 1900o C. The reactions taking place in
converter are as follows:
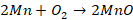
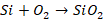
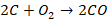
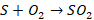
The
oxides of silicon and manganese combine to form a slag of manganese silicate
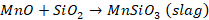
A
little amount of iron is also oxidized to ferric oxide, but it gets readily
reduced by carbon present in the cast iron.
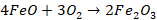
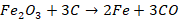
Ferric
oxide formed above also oxidizes manganese and silicon to their respective
oxides
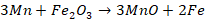
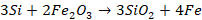
These reactions
are then observed by the colour of burning waste gases. Carbon monoxide
produced burns at the mouth of the converter with a blue flame and orange-red
tinge, throwing out shower of sparks. When whole carbon is oxidized, the blue
flame suddenly dies down. When the blue flame dies, a calculated amount of Fe, Mn,
and C is added.
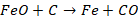
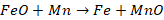
Blast
of air is passed; mass is mixed well. Molten iron contains dissolved gases such
as O2, N2 and CO2 which create blow holes or
gas bubbles in castings and defects are created. This is then followed by the
addition of aluminium. Aluminium reacts with these gases and removes them as
slag.
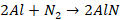
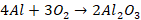
Basic Bessemer
This
process is used to treat cast iron containing phosphorus. In this process, the
converter is lined with magnesia and lime prepared by calcinations of dolomite (CaCO3.MgCO3).
Some limestone is added into the converter. Molten cast iron from blast furnace
is then run into the converter and the blast is continued. Carbon, sulphur, and
manganese are oxidized first as usual, but if the blast is continued even after
the flame sinks down, then the phosphorus forms phosphorus pentoxide.
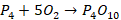
The
phosphorus pentoxide thus formed, combines with lime to form a basic slag,
containing calcium phosphate
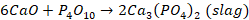
After
complete removal of slag, required amount of carbon is added and the product is
thoroughly mixed in presence of air.
Merits of Bessemer Process
ü This
process is useful for rapid production of steel.
ü Cost
of operating process is low
ü No
extra fuel is required as molten cast iron is directly taken to converter from
blast furnace.
Demerits of Bessemer Process
ü Steel
produced is of inferior quality
ü Approx
15% of iron is lost in slag
ü Process
is not continuous and charging is tedious
Linz-Donawitz Process
LD is
named after the two places of Austria namely, Linz and Donawitz where the
process was first formed. It is a refining process which is done through an LD
convertor or LD vessel. LD is also known as Basic Oxygen Process. The furnace
is an egg-shaped steel vessel, sealed from bottom and supported on the side
arms called trunnions. It is lined from inside with basic lining of magnetise
or limestone. At the top, it also has an oxygen lance made up of concentric
steel tubes with a tip of copper.
Charging: Five materials are required
for this purpose. The converter is charged with cast iron, scrap, limestone,
coolants, pure and dry oxygen.
Blowing: Once the charging is done then
the converter is rotated upright in the vertical position and the lance is lowered
to the position of blowing. O2 is then turned on for around 20
minutes, at a pressure of 9 to 11 atoms that raises the temperature and
impurities are burned off.
Sampling: for analysis, slag and metal
samples can be taken and the temperature of the bath needs to be measured by
immersion of thermocouple.
Tapping: the
molten steel is tapped in the ladle if the tapping temperature is in the
required range. Ladle is used to make deoxidizers and alloying additions. It
has a tap-to-tap time of 40-50mins.
Slag off: after
tapping steel into the ladle and putting the vessel upside down tapped the
remaining slag into the slag pot.
Merits
ü It
is rapid process with high productivity.
ü Energy
needed is comparatively low.
ü Steel
produced is of superior quality.
ü Molten
cast iron and scrap can be used directly.
Composition, properties, and application of plain carbon steel
Plain
carbon steel is an alloy of iron and carbon where the amount of carbon ranges
from, 0.015% to 2%. Carbon steel is by far the most widely used kind of steel.
The properties of carbon steel depend primarily on the amount of carbon it
contains. In fact, there are 3 types of plain carbon steel and they are low
carbon steel, medium carbon steel, high carbon steel, and as their names
suggests all these types of plain carbon steel differs in the amount of carbon
they contain. Indeed, it is good to precise that plain carbon steel is a type
of steel having a maximum carbon content of 1.5% along with small percentages
of silica, sulphur, phosphorus and manganese.
Plain
carbon steels are further subdivided into four groups:
Low carbon steels (carbon: 0.05% <carbon< 0.30%):
Often called mild steels, low-carbon steels are the most commonly used grades.
They machine and weld nicely and are more ductile than higher-carbon steels.
Use: It is used for thin soft
wires, ropes, chains, tubes, etc.
Medium carbon steels (0.30% < carbon < 0.60%):
Increased carbon means increased hardness and tensile strength, decreased
ductility, and more difficult machining.
Use: It
is mainly used in rail roads, wheels, axles, crane hooks, etc.
High carbon steels (0.6% < carbon < 0.90%):
these steels can be challenging to weld. Preheating, post heating (to control
cooling rate), and sometimes even heating during welding become necessary to
produce acceptable welds and to control the mechanical properties of the steel
after welding.
Use: It is used for making tools,
drills, knives, hammers, etc.
Very high carbon steels (0.9% < carbon < 1.5%):
very high-carbon steels are used for hard steel products such as metal cutting
tools and truck springs. Like high-carbon steels, they require heat treating
before, during, and after welding to maintain their mechanical properties.
Use: It
is mainly used for preparation of cutting tools, blades, saws, etc.
Limitations of plain carbon steel:
ü There
cannot be strengthening beyond about 100000 psi without significant loss in
toughness (impact resistance) and ductility.
ü Plain-carbon
steels have poor impact resistance at low temperatures.
ü Plain-carbon
steels have poor corrosion resistance for engineering problems.
ü Plain-carbon
steel oxidises readily at elevated temperatures.
Effects of various alloying elements on steel
Steel
is alloyed with various elements to improve physical properties and to produce
special properties such as resistance to corrosion or heat. Steel is alloyed
with number of elements such as Nickel, Silicon, Chromium, etc. Specific
effects of the addition of such elements are outlined below:
Some
important alloy steels have been listed:
|
Name
|
Composition
|
Properties
|
Uses
|
|
Molybdenum
Steel
|
0.3%
to 3% Mo
|
Increases
strength, hardness, hardenability, and toughness and strength even at high
temperatures. It improves machinability and resistance to corrosion.
|
Axis and
cutting tools
|
|
Nickel
steel platinate
|
46%
Ni
|
Increases
strength and hardness without sacrificing ductility and toughness. High
co-efficient of expansion
|
Wire
scales, electric bulbs, etc.
|
|
Chrome
steels
|
2%
to 4% Cr
|
Increases
tensile strength, hardness, hardenability, toughness, resistance to wear and
abrasion
|
Ball
bearings, cutting tools, etc.
|
|
Manganese
steels
|
6%
to 15% Mn
|
It
increases tensile strength, hardness, hardenability and resistance to wear.
|
Crushing
Machine, helmet, railroad, etc.
|
|
Stainless
steel
|
12%
to 20% Cr, 8% Ni
|
Resistant
to corrosion, non-magnetic, brittle.
|
Cutlery,
utensils, surgical appliances
|
|
Nickel
Steel
|
2.5%
to 5% Ni
|
Hard,
resistant to corrosion
|
Wires,
cables, gears, etc.
|
|
Cobalt
steel
|
Upto
35% Co
|
High
magnetism, hard, resistant to corrosion
|
High
speed tools and permanent magnets
|
|
Silicon
steel
|
Upto
15% Si
|
Extremely
hard, resistant to acids
|
Pumps,
pipes carrying acids, transformers
|
|
Tungsten
steel
|
15%
to 20% W
|
Increases
strength, wear resistance, hardness and toughness. Tungsten steels have
superior hot-working and greater cutting efficiency at elevated temperatures.
|
High
speed machine, drilling tools, cutting tools, etc.
|
|
Vanadium
steel
|
3.5%
to 5% V
|
Increases
strength, hardness, wear resistance and resistance to shock impact. It
retards grain growth, permitting higher quenching temperatures
|
Cutting
tools
|
Non-ferrous alloys
Non-ferrous
metals are alloys or metals that do not contain any appreciable amounts of iron.
Non-ferrous alloys are formed by mixing atoms of transition metal other than
iron with a non-transition metal. Example: Brass is an alloy of copper and
zinc. It does not contain iron and hence it is non-ferrous alloy. Several non-ferrous
alloys are discussed below:
Copper Alloys
ü Alloys
which contain copper as base metal are known as copper alloys.
Properties of copper alloy
ü Copper
alloys are generally reddish in colour.
ü Its
specific gravity is 8.93 and hence it is a heavy metal.
ü It
has high melting and boiling point.
ü Copper
alloys are generally good conductor of electricity and heat
ü Copper
alloys are malleable, tough and ductile
ü Copper
alloys are resistant to corrosion
Composition of copper alloy
|
Name
|
Composition
|
Property
|
Application
|
|
Brass
|
66%
Cu
34%
Zn
|
Bright gold
appearance. Highly ductile, low friction and resistant to corrosion
|
Bells,
horns, utensil, jewellery
|
|
Bronze
|
88%
Cu
12%
Tin
|
Hard and brittle.
Low metal to metal friction. High resistance to corrosion from saltwater
|
Used
in sculpture, musical instruments
|
|
Nickel
silver or German silver
|
18%
Ni
62%
Cu
20%
Zn
|
Silver
white appearance Highly lustrous, tough and corrosion resistance
|
Cutlery,
marine fittings, heating coils
|
Aluminium alloys
An
aluminium alloy is an alloy in which aluminium (Al) is the predominant metal.
The typical alloying elements are copper, magnesium, manganese, etc.
Properties of aluminium alloy
ü Aluminium
alloys are generally bluish white
ü Its
specific gravity is 2.7 and hence it is a light metal
ü It
has comparatively low melting point and high boiling point
ü It
is malleable and ductile
ü Aluminium
alloys are generally good reflector of light
Composition of aluminium alloy
|
Name
|
Composition
|
Property
|
Application
|
|
Duralumin
|
94%
Al
4%
Cu
1%
Mg
0.5-1%
Mn
|
Whitish
appearance, resistant to corrosion, hard, good conductor of heat and
electricity
|
Wire,
bar, rods, wheels, aircraft fittings, etc.
|
|
Magnalium
|
95%
Al
5%
Mg
|
Light
weight and brittle. Poor castability and good machinability
|
Suitable
for making aircraft and automobile parts.
|
Solder alloys
Solder
alloy is a metallic material that is used to connect metal workpieces. Solder
alloys are generally alloys of lead and tin and has low melting point. The
solder alloy becomes softer as the percentage of lead increases and harder as percentage
of tin increases. Solder alloys easily adhere to the metallic surface.
Some more alloys
|
Name
|
Composition
|
Properties
|
Uses
|
|
Soft
solder
|
37%-67%
Lead
31.60%
Tin
0.12%
Stibium
|
Melt
at low temperatures. Percentage of lead is more and hence it is a soft solder
|
Used
for soldering electrical connections.
|
|
Tinman’s
solder
|
66%
Tin
34%
lead
|
It
melts at 180o C. Percentage of lead is less and hence it is a hard
solder
|
Used
for soldering tin articles
|
|
Plumber’s
solder
|
67%
lead
33%
tin
|
Superior
wetting and capillary filling characteristics
|
Used
in making wiped joints and seams
|
|
Brazing
alloys
|
92%
tin
5.5%
lead
2.5%
Copper
|
It
melts at comparatively higher temperature
|
Generally
used for steel joints.
|
|
Rose
Metal
|
50%
Bismuth
25%-28%
lead
22-25%
tin
|
It
is a fusible alloy. Very low melting point
|
Used
for making fire alarms, dental works, automatic sprinkler system
|
|
Wood’s
metal
|
50%
Bismuth
26%
lead
12.5%
Tin
12.5%
Cadmium
|
Very
low melting point. It is very dense and easily fusible
|
Joining
two metallic parts, fire alarms, electric fuse wires.
|